stdClass Object
(
[nazev] => Department of Organic Chemistry
[adresa_url] =>
[api_hash] =>
[seo_desc] =>
[jazyk] =>
[jednojazycny] =>
[barva] =>
[indexace] => 1
[obrazek] =>
[ga_force] =>
[cookie_force] =>
[secureredirect] =>
[google_verification] => UOa3DCAUaJJ2C3MuUhI9eR1T9ZNzenZfHPQN4wupOE8
[ga_account] => UA-10822215-3
[ga_domain] =>
[ga4_account] => G-VKDBFLKL51
[gtm_id] =>
[gt_code] =>
[kontrola_pred] =>
[omezeni] => 0
[pozadi1] =>
[pozadi2] =>
[pozadi3] =>
[pozadi4] =>
[pozadi5] =>
[robots] =>
[htmlheaders] =>
[newurl_domain] => 'uoch.vscht.cz'
[newurl_jazyk] => 'en'
[newurl_akce] => '[en]'
[newurl_iduzel] =>
[newurl_path] => 8548/6214/6521
[newurl_path_link] => Odkaz na newurlCMS
[iduzel] => 6521
[platne_od] => 31.10.2023 17:15:00
[zmeneno_cas] => 31.10.2023 17:15:57.546654
[zmeneno_uzivatel_jmeno] => Jan Kříž
[canonical_url] =>
[idvazba] => 7334
[cms_time] => 1713441950
[skupina_www] => Array
(
)
[slovnik] => stdClass Object
(
[logo_href] => /
[logo] =>  [google_search] => 001523547858480163194:u-cbn29rzve
[social_fb_odkaz] => https://www.facebook.com/uoch.vscht
[social_tw_odkaz] => https://twitter.com/uoch_vscht
[social_yt_odkaz] =>
[paticka_budova_a_nadpis] => BUILDING A
[paticka_budova_a_popis] => Rector,
Department of Communications,
Department of Education,
FCT Dean’s Office,
Centre for Information Services
[paticka_budova_b_nadpis] => BUILDING B
[paticka_budova_b_popis] => Department of R&D, Dean’s Offices:
FET,
FFBT,
FCE,
Computer Centre,
Department of International Relations,
Bursar
[paticka_budova_c_nadpis] => BUILDING C
[paticka_budova_c_popis] =>
Crèche Zkumavka,
General Practitioner,
Department of Economics and Management,
Department of Mathematics
[paticka_budova_1_nadpis] => NATIONAL LIBRARY OF TECHNOLOGY
[paticka_budova_1_popis] =>
[paticka_budova_2_nadpis] => CAFÉ CARBON
[paticka_budova_2_popis] =>
[paticka_adresa] => UCT Prague
[google_search] => 001523547858480163194:u-cbn29rzve
[social_fb_odkaz] => https://www.facebook.com/uoch.vscht
[social_tw_odkaz] => https://twitter.com/uoch_vscht
[social_yt_odkaz] =>
[paticka_budova_a_nadpis] => BUILDING A
[paticka_budova_a_popis] => Rector,
Department of Communications,
Department of Education,
FCT Dean’s Office,
Centre for Information Services
[paticka_budova_b_nadpis] => BUILDING B
[paticka_budova_b_popis] => Department of R&D, Dean’s Offices:
FET,
FFBT,
FCE,
Computer Centre,
Department of International Relations,
Bursar
[paticka_budova_c_nadpis] => BUILDING C
[paticka_budova_c_popis] =>
Crèche Zkumavka,
General Practitioner,
Department of Economics and Management,
Department of Mathematics
[paticka_budova_1_nadpis] => NATIONAL LIBRARY OF TECHNOLOGY
[paticka_budova_1_popis] =>
[paticka_budova_2_nadpis] => CAFÉ CARBON
[paticka_budova_2_popis] =>
[paticka_adresa] => UCT Prague
Technická 5
166 28 Prague 6 – Dejvice
IČO: 60461373 / VAT: CZ60461373
Czech Post certified digital mail code: sp4j9ch
Copyright: UCT Prague 2015
Information provided by the Department of Organic Chemistry. Technical support by the Computing Centre.
[paticka_odkaz_mail] => mailto:kundrato@vscht.cz
[social_fb_title] => Facebook of Department
[social_tw_title] => Twitter of Department
[social_yt_title] =>
[aktualizovano] => Updated
[autor] => Author
[paticka_mapa_alt] =>
[drobecky] => You are here: VŠCHT Praha – FCHT – ÚOCH
[intranet_odkaz] => http://intranet.vscht.cz/
[intranet_text] => Intranet
[logo_mobile_href] => /
[logo_mobile] =>  [mobile_over_nadpis_menu] => Menu
[mobile_over_nadpis_search] => Search
[mobile_over_nadpis_jazyky] => Languages
[mobile_over_nadpis_login] => Login
[menu_home] => Homepage
[zobraz_desktop_verzi] => switch to desktop version
[zobraz_mobilni_verzi] => switch to mobile version
[more_info] => more information
[paticka_mapa_odkaz] =>
[nepodporovany_prohlizec] => For full access, please use different browser.
[preloader] => Wait a second...
[social_in_odkaz] =>
[den_kratky_4] =>
[archiv_novinek] =>
[novinky_servis_archiv_rok] =>
[novinky_kategorie_1] =>
[novinky_kategorie_2] =>
[novinky_kategorie_3] =>
[novinky_kategorie_4] =>
[novinky_kategorie_5] =>
[novinky_archiv_url] =>
[novinky_servis_nadpis] =>
[novinky_dalsi] =>
[hledani_nadpis] => hledání
[hledani_nenalezeno] => Nenalezeno...
[hledani_vyhledat_google] => vyhledat pomocí Google
[social_li_odkaz] =>
)
[poduzel] => stdClass Object
(
[6525] => stdClass Object
(
[obsah] =>
[poduzel] => stdClass Object
(
[6532] => stdClass Object
(
[obsah] =>
[iduzel] => 6532
[canonical_url] => //uoch.vscht.cz
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
[6530] => stdClass Object
(
[obsah] =>
[iduzel] => 6530
[canonical_url] => //uoch.vscht.cz
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
[6531] => stdClass Object
(
[obsah] =>
[iduzel] => 6531
[canonical_url] => //uoch.vscht.cz
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
)
[iduzel] => 6525
[canonical_url] =>
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
[6526] => stdClass Object
(
[obsah] =>
[poduzel] => stdClass Object
(
[21923] => stdClass Object
(
[nazev] => Department of Organic Chemistry
[seo_title] => Department of Organic Chemistry
[seo_desc] =>
[autor] =>
[autor_email] =>
[obsah] =>
[mobile_over_nadpis_menu] => Menu
[mobile_over_nadpis_search] => Search
[mobile_over_nadpis_jazyky] => Languages
[mobile_over_nadpis_login] => Login
[menu_home] => Homepage
[zobraz_desktop_verzi] => switch to desktop version
[zobraz_mobilni_verzi] => switch to mobile version
[more_info] => more information
[paticka_mapa_odkaz] =>
[nepodporovany_prohlizec] => For full access, please use different browser.
[preloader] => Wait a second...
[social_in_odkaz] =>
[den_kratky_4] =>
[archiv_novinek] =>
[novinky_servis_archiv_rok] =>
[novinky_kategorie_1] =>
[novinky_kategorie_2] =>
[novinky_kategorie_3] =>
[novinky_kategorie_4] =>
[novinky_kategorie_5] =>
[novinky_archiv_url] =>
[novinky_servis_nadpis] =>
[novinky_dalsi] =>
[hledani_nadpis] => hledání
[hledani_nenalezeno] => Nenalezeno...
[hledani_vyhledat_google] => vyhledat pomocí Google
[social_li_odkaz] =>
)
[poduzel] => stdClass Object
(
[6525] => stdClass Object
(
[obsah] =>
[poduzel] => stdClass Object
(
[6532] => stdClass Object
(
[obsah] =>
[iduzel] => 6532
[canonical_url] => //uoch.vscht.cz
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
[6530] => stdClass Object
(
[obsah] =>
[iduzel] => 6530
[canonical_url] => //uoch.vscht.cz
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
[6531] => stdClass Object
(
[obsah] =>
[iduzel] => 6531
[canonical_url] => //uoch.vscht.cz
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
)
[iduzel] => 6525
[canonical_url] =>
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
[6526] => stdClass Object
(
[obsah] =>
[poduzel] => stdClass Object
(
[21923] => stdClass Object
(
[nazev] => Department of Organic Chemistry
[seo_title] => Department of Organic Chemistry
[seo_desc] =>
[autor] =>
[autor_email] =>
[obsah] => New PhD topics of our department: here
All publications of our authors (since 2015): here
[urlnadstranka] =>
[ogobrazek] =>
[pozadi] =>
[iduzel] => 21923
[canonical_url] =>
[skupina_www] => Array
(
)
[url] => /home
[sablona] => stdClass Object
(
[class] => boxy
[html] =>
[css] =>
[js] => $(function() {
setInterval(function () { $('*[data-countdown]').each(function() { CountDownIt('#'+$(this).attr("id")); }); },1000);
setInterval(function () { $('.homebox_slider:not(.stop)').each(function () { slide($(this),true); }); },5000);
});
function CountDownIt(selector) {
var el=$(selector);foo = new Date;
var unixtime = el.attr('data-countdown')*1-parseInt(foo.getTime() / 1000); if(unixtime<0) unixtime=0;
var dnu = 1*parseInt(unixtime / (3600*24)); unixtime=unixtime-(dnu*(3600*24));
var hodin = 1*parseInt(unixtime / (3600)); unixtime=unixtime-(hodin*(3600));
var minut = 1*parseInt(unixtime / (60)); unixtime=unixtime-(minut*(60));
if(unixtime<10) {unixtime='0'+unixtime;}
if(dnu<10) {unixtime='0'+dnu;}
if(hodin<10) {unixtime='0'+hodin;}
if(minut<10) {unixtime='0'+minut;}
el.html(dnu+':'+hodin+':'+minut+':'+unixtime);
}
function slide(el,vlevo) {
if(el.length<1) return false; var leva=el.find('.content').position().left; var sirka=el.width(); var pocet=el.find('.content .homebox').length-1;
var cislo=leva/sirka*-1; if(vlevo) { if(cislo+1>pocet) cislo=0; else cislo++; } else { if(cislo==0) cislo=pocet-1; else cislo--; }
el.find('.content').animate({'left':-1*cislo*sirka});
el.find('.slider_puntiky a').removeClass('selected');
el.find('.slider_puntiky a.puntik'+cislo).addClass('selected');
return false;
}
function slideTo(el,cislo) {
if(el.length<1) return false; var sirka=el.width(); var pocet=el.find('.content .homebox').length-1; if(cislo<0 || cislo>pocet) return false;
el.find('.content').animate({'left':-1*cislo*sirka});
el.find('.slider_puntiky a').removeClass('selected');
el.find('.slider_puntiky a.puntik'+cislo).addClass('selected');
return false;
}
[autonomni] => 1
)
)
[16022] => stdClass Object
(
[nazev] => Department of Organic Chemistry - Staff
[seo_title] => Department of Organic Chemistry
[seo_desc] =>
[autor] =>
[autor_email] =>
[perex] =>
[ikona] => telefon-zvoni
[obrazek] =>
[ogobrazek] =>
[pozadi] =>
[obsah] =>
| k Phonebook of the Department | |||
Professors: |
|||
|
Prof. Radek Cibulka e +420 22044 4182 d A 278c |
Head of Department | www | ResearcherID |
|
Prof. Pavel Lhoták e +420 22044 5055 d A 249 |
www | ||
|
Prof. Jaroslav Kvíčala e +420 22044 4240 d A 278d |
www | ||
|
Prof. Jiří Svoboda e +420 22044 3688 d A 278e |
www | ||
Associate Professors: |
|||
|
Assoc. Prof. Jan Budka e +420 22044 4284 d A 251 |
DOC web editor | www | |
|
Assoc. Prof. Jana Hodačová e +420 22044 4173 d A 270 |
www | ||
|
Assoc. Prof. Michal Kohout e +420 22044 5012 d A 258 |
www | ||
|
Assoc. Prof. Igor Linhart e +420 22044 4165 d A 266 |
www | ||
|
Assoc. Prof. Tomáš Tobrman e +420 22044 4245 d A 271 |
RIT | www | |
Assistant Professors: |
|||
|
Dr. Michal Himl e +420 22044 4165 d A 266 |
www | ||
|
Dr. Roman Holakovský e +420 22044 4279 d A 255 |
www | ||
|
Dr. Petr Kovaříček e +420 22044 2040 d B3 207 |
www | ||
|
Dr. Václav Kozmík e +420 22044 4118 d A 308 |
www | ||
|
Dr. Martin Krupička e +420 220 444 173 |
www | ||
|
Dr. Ondřej Kundrát e +420 22044 4280 d A 253 |
www | ResearcherID | |
|
Dr. Petra Ménová e +420 22044 3686 d A 268 |
www | ||
|
Dr. Pavla Perlíková e +420 22044 2039 d B3 206 |
www | ||
|
Dr. Markéta Rybáčková e +420 22044 4242 d A 259 |
Secretary of Department |
www | |
|
Dr. Eva Svobodová e +420 22044 4249 d A 260 |
www | ||
Technicians: |
|||
|
Květa Bártová e +420 22044 3686 d A 268 |
|||
|
Ivana Bocková, MSc. e +420 22044 4280 d A 250 |
|||
| Michaela Kadlecová
e +420 22044 4276 d A 262 b Michaela.Kadlecova@vscht.cz |
|||
|
Martina Kovandová e +420 22044 4279 d A 255 |
|||
|
Vladimír Kuneš e +420 22044 4277 d A L06 |
|||
|
Jana Netušilová e +420 22044 4164 d A 278a |
Administrative Support of Department | ||
|
Markéta Slabochová, MSc. e +420 22044 4059 d A 308 |
Economist of Department | ||
|
Helena Štenglová e +420 22044 4278 d A 257 |
|||
|
Ing. David Tetour e +420 22044 4173 d A270 |
|||
|
Marta Tokárová e +420 220 444 245 d A 271 |
|||
Essential Information for Current Students
Bachelor Students
Master Students
PhD Students
Lectures delivered by members of the Department
- Toxicology and ecology
- Organic chemistry I, II and III
- Structural analysis
- Reactivity of organic compounds
- Molecular design
- Organic synthesis I and II
- Quantum organic chemistry
- Farmacochemistry
- Organic stereochemistry
- Organometallic chemistry
- Organic chemistry of selected elements
DATA
stdClass Object
(
[nazev] =>
[seo_title] => Cibulka
[seo_desc] =>
[autor] =>
[autor_email] =>
[obsah] => 
Prof. Radek Cibulka, Ph.D.
Chemistry_Europe_Fellows Class 2020/21

Adam Pokluda - IGRA
As a co-researcher, Adam Pokluda from our laboratory will participate on the prestigious IGRA grant at the University of Chemistry and Technology, Prague entitled "Alloxazine photon catching antenna for molecular switches in organic electronics". The main researcher is Marek Čubiňák from the Institute of Organic Chemistry. The Institute of Physical Chemistry also participates in the project.
Paper in a prestigious journal:
Graml A., Neveselý T., Kutta R.-J., Cibulka R., König B.: Deazaflavin reductive photocatalysis involves excited semiquinone radicals Nature Comm. 2020, 11, 3174. DOI: 10.1038/s41467-020-16909-y. https://rdcu.be/b47PV
News and views paper in Nature:
Cibulka R.: Strong chemical reducing agents produced by light Nature 580, 31-32 (2020). DOI:10.1038/d41586-020-00872-1. https://www.nature.com/articles/d41586-020-00872-1
Highly cited papers
Article (Org. Lett. 2019, 21, 114-119) published by our group in cooperation with the group of prof. Roithová met the criteria of web of science for "Highly cited papers“ (As of November/December 2019, this paper received enough citations to place it in the top 1% of the academic field of Chemistry based on a highly cited threshold for the field and publication year).
Abstract:
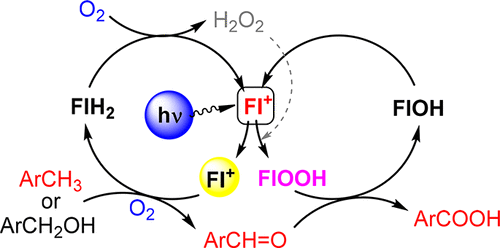
We report a system with ethylene-bridged flavinium salt 2b which catalyzes the aerobic oxidation of toluenes and benzyl alcohols with high oxidation potential (Eox > +2.5 V vs SCE) to give the corresponding benzoic acids under visible light irradiation. This is caused by the high oxidizing power of excited 2b (E(2b*) = +2.67 V vs SCE) involved in photooxidation and by the accompanying dark organocatalytic oxygenation provided by the in situ formed flavin hydroperoxide 2b-OOH.
DOI:10.1021/acs.orglett.8b03547.
[submenuno] =>
[urlnadstranka] =>
[ogobrazek] =>
[pozadi] =>
[newurl_domain] => 'uoch.vscht.cz'
[newurl_jazyk] => 'en'
[newurl_akce] => '/research-groups/cibulka-en'
[newurl_iduzel] => 7839
[newurl_path] => 8548/6214/6521/6526/7837/7839
[newurl_path_link] => Odkaz na newurlCMS
[iduzel] => 7839
[platne_od] => 01.01.2024 20:39:00
[zmeneno_cas] => 01.01.2024 20:39:24.300523
[zmeneno_uzivatel_jmeno] => Martina Kovandová
[canonical_url] =>
[idvazba] => 8894
[cms_time] => 1713443729
[skupina_www] => Array
(
)
[slovnik] => Array
(
)
[poduzel] => stdClass Object
(
[38649] => stdClass Object
(
[nazev] => Cibulka's Group
[barva_pozadi] => cervena
[uslideru] => false
[text] =>
[poduzel] => Array
(
)
[iduzel] => 38649
[canonical_url] =>
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] => infobox
[html] =>
[css] =>
[js] =>
[autonomni] => 0
)
)
[53752] => stdClass Object
(
[obsah] =>
[poduzel] => stdClass Object
(
[53753] => stdClass Object
(
[obsah] =>
[iduzel] => 53753
[canonical_url] =>
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] => novinka
[html] =>
[css] =>
[js] =>
[autonomni] => 0
)
)
)
[iduzel] => 53752
[canonical_url] =>
[skupina_www] => Array
(
)
[url] => /research-groups/cibulka-en/53752
[sablona] => stdClass Object
(
[class] => novinky
[html] =>
[css] =>
[js] =>
[autonomni] => 1
)
)
[39232] => stdClass Object
(
[obsah] =>
[poduzel] => stdClass Object
(
[39233] => stdClass Object
(
[nadpis] =>
[popis] =>
[platne_od] =>
[platne_do] =>
[odkaz] =>
[text_odkazu] =>
[obrazek_pozadi] => 0001~~K87JTEktUkjOTCrNyU5USM0DAA.png
[barva_textu] =>
[iduzel] => 39233
[canonical_url] =>
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] => slider
[html] =>
[css] =>
[js] =>
[autonomni] => 0
)
)
[53750] => stdClass Object
(
[nadpis] =>
[popis] =>
[platne_od] =>
[platne_do] =>
[odkaz] =>
[text_odkazu] =>
[obrazek_pozadi] => 0001~~y8lMSY03AQA.png
[barva_textu] =>
[iduzel] => 53750
[canonical_url] =>
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] => slider
[html] =>
[css] =>
[js] =>
[autonomni] => 0
)
)
)
[iduzel] => 39232
[canonical_url] =>
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] => slider
[html] =>
[css] =>
[js] =>
[autonomni] => 0
)
)
[39227] => stdClass Object
(
[nazev] => Contacts
[barva_pozadi] => cervena
[uslideru] => false
[text] => Prof. Radek Cibulka
building A, room 278c
k + 420 220 44 4182
b Radek.Cibulka@vscht.cz
Dr. Roman Holakovský
building A, room 255
k + 420 220 44 4279
b Roman.Holakovsky@vscht.cz
Dr. Eva Svobodová
building A, room 260
k + 420 220 44 4249
+ 420 220 44 4279
b Eva.Svobodova@vscht.cz
[poduzel] => Array ( ) [iduzel] => 39227 [canonical_url] => [skupina_www] => Array ( ) [url] => [sablona] => stdClass Object ( [class] => infobox [html] => [css] => [js] => [autonomni] => 0 ) ) [7840] => stdClass Object ( [nazev] => [seo_title] => People [seo_desc] => [autor] => [autor_email] => [obsah] =>
Employees:Ph.D. students:
Students:
Students Collaborations:
Collaborations:
Guests:
|
Curriculum Vitae
Prof. Radek Cibulka, Ph.D.
(ID: H-1298-2016, ORCID ID: 0000-0002-8584-7715)
Personal data:
Born: 12. 3. 1973 in Sokolov, Czech Republic
Marital status: married, 3 children (born in 2000, 2002, and 2007)
Education: M.Sc.: University of Chemistry and Technology, Prague (UCT Prague) (1996)
Ph.D.: UCT Prague (2002)
doc.: habilitation in Organic Chemistry, UCT Prague (2009)
prof.: professor in Organic Chemistry, UCT Prague (2017)
Career summary:
1991-1996 Grammar school in Sokolov
1996 M.Sc. with F. Hampl, Department of Organic Chemistry, University of Chemistry and Technology, Prague (UCT Prague), Czech Republic
2002 Ph.D. with F. Liška, Department of Organic Chemistry, UCT Prague, Czech Republic
2003 post-doc with B. König, Department of Organic Chemistry, University of Regensburg, Germany
2004 Assistant professor, Department of Organic Chemistry, UCT Prague, Czech Republic
2009 Associate Professor, Department of Organic Chemistry, UCT Prague, Czech Republic
since 2017 Full professor, Department of Organic Chemistry, UCT Prague, Czech Republic
Awards:
1996 Prize of Josef Hlavka from Hlavka’s Foundation
2002 Unipetrol Prize from Unipetrol and University of Chemistry and Technology, Prague
2004 Young Chemistry Award in Organic Chemistry from Sigma-Aldrich in the Czech Republic
2005 Alfred Bader Prize for Organic Chemistry from the Czech Chemical Society
2010 Karel Preis Prize for the best article in Chemické listy from the Czech Chemical Society
Expertise:
Reviewer of manuscripts submitted for: Nature, Nature comm., Org. Biomol. Chem., Chem. Comm., Chem. Sci., Angew. Chem., Chemistry A European Journal etc.
Evaluation of projects for Grant agency of Charles University, Prague; Ministry of Youth, Sports and Education of the Czech Republic, Experientia foundation
Activities in organic chemistry and catalysis since 2015:
Research interests; Photocatalysis; Organocatalysis in redox reactions; Flavin based redox and photoactive systems; Heterocyclic chemistry
Selected projects:
2012 – 2015 Organocatalytic stereoselective oxidations using heterocyclic hydroperoxides (principle investigator; Czech Science Foundation; No P207/12/0447)
2014 – 2016 Towards photoorganocatalytic metathesis: [2+2] cycloaddition of alkenes and cyclobutane ring cleavage mediated by flavins and visible light (principle investigator; Czech Science Foundation; No 14-09190S)
2016 – 2018 Photocatalytic and organocatalytic Mitsunobu reaction using flavin derivatives and visible light (principle investigator; Czech Science Foundation; No 16-09436S)
2018 – 2020 Oxidative photocatalytic systems with flavinium salts for direct C-H functionalizations (principle investigator; Czech Science Foundation; No 18-15175S)
2019 – 2021 Organic photoredox catalysis in reductive transformations: new area for flavin derivatives (principle investigator; Czech Science Foundation; No 19-09064S)
Education:
Lectures in Retrosynthesis (2011-now); Organic chemistry (2011-now); courses in Laboratory of organic chemistry (2011 – 2015); co-author of internet portal for education of organic chemistry (2011 – 2013); Supervisor of 10 Ph.D. thesis (6 finished, 4 ongoing), 21 diploma thesis and 21 bachelor thesis.
Publications - summary:
62 original papers, 5 patents, 4 review articles, 2 book chapters, 140 oral or poster presentations,
24 plenary or invited lectures; h = 20.
5 Selected papers from the last 5 years:
Mojr V., Svobodová E., Straková K., Neveselý T., Chudoba J., Dvořáková H., Cibulka R.: Tailoring Flavins for Visible Light Photocatalysis: Organocatalytic [2+2] Cycloadditions Mediated by a Flavin Derivative and Visible Light, Chem. Comm. 2015, 51, 12036 – 12039.
Neveselý T., Svobodová E., Chudoba J., Sikorski M., Cibulka R.: Efficient metal-free aerobic photooxidation of sulfides to sulfoxides mediated by a vitamin B2 derivative and visible light, Adv. Synth. Catal. 2016, 358, 1654–1663. (VIP)
Hartman T., Cibulka R.: Photocatalytic Systems with Flavinium Salts: From Photolyase Models to Synthetic Tool for Cyclobutane Ring Opening, Org. Lett. 2016, 18, 3710-3713.
März M., Kohout M., Neveselý T., Chudoba J., Prukala D., Niziński S., Sikorski M., Burdziński G., Cibulka R.: Azodicarboxylate-free esterification with triphenylphosphine mediated by flavin and visible light: method development and stereoselectivity control Org. Biomol. Chem. 2018, 16, 6809-6817. (Hot articles collection)
Zelenka J., Svobodová E., Tarábek J., Hoskovcová I., Boguschová V., Bailly S., Sikorski M., Roithová J., Cibulka R.: Combining flavin photocatalysis and organocatalysis: metal-free aerobic oxidation of unactivated benzylic substrates Org. Lett. 2019, 21, 114-119.
Zelenka J., Cibulka R., Roithová J.: Flavinium catalyzed photooxidation: Detection and characterization of elusive peroxyflavinium intermediates Angew. Chem. Int. Ed. 2019, 58, 15412 –15420. (VIP)
Contact:
Address: Radek Cibulka, Department of Organic Chemistry, University of Chemistry and Technology, Prague, Technická 5, 16628 Prague 6, Czech Republic
Phone number: +420 220 444 182
E-mail: cibulkar@vscht.cz
In Prague, February 14 2020
[urlnadstranka] => [obrazek] => [pozadi] => [iduzel] => 39224 [canonical_url] => [skupina_www] => Array ( ) [url] => /research-groups/cibulka-en/people/39224 [sablona] => stdClass Object ( [class] => stranka [html] => [css] => [js] => [autonomni] => 1 ) ) [39226] => stdClass Object ( [nazev] => Dr. Eva Svobodová [seo_title] => Dr. Eva Svobodová [seo_desc] => Dr. Eva Svobodová [autor] => [autor_email] => [obsah] =>
 Curriculum Vitae
Curriculum Vitae
Personal data:
Name: Ing. Eva Svobodová, Ph.D.
Born: 18.1.1978 in Vsetín, Czech Republic
Marital status: married, 2 children (born in 2008 and 2010)
e-mail: svobodoe@vscht.cz
Career Summary:
1992-1996 Grammar school in Vsetín, Czech Republic
1996-2001 M.Sc. with F. Liška, University of Chemistry and Technology, Prague, Czech Republic
2001-2006 Ph.D. with F. Hampl, University of Chemistry and Technology, Prague, Czech Republic
Since 2006 Assistant professor, Department of Organic Chemistry, University of Chemistry and Technology, Prague. Czech Republic
Awards:
The Foundation CURIE BRATISLAVA SIS´03 Award - the best student presentation on 10th International conference Separation of Ionic Solutes, Podbanské, Vysoké Tatry, Slovakia, 6 – 11. 9. 2003.
Teaching Activity:
Lectures in Organic chemistry, courses in Laboratory of organic chemistry.
Supervising of bachelor thesis.
Research Interest:
Chemistry of heterocyclic compounds, flavins and their derivatives, photocatalysis
Publication Activity:
10 original papers in impacted journals, 1 patent, 24 presentations on conferences (author or co-author)
[iduzel] => 39226 [canonical_url] => //uoch.vscht.cz/research-groups/cibulka-en/people/39226 [skupina_www] => Array ( ) [url] => /research-groups/cibulka-en/people/39226 [sablona] => stdClass Object ( [class] => stranka [html] => [css] => [js] => [autonomni] => 1 ) ) ) [iduzel] => 7840 [canonical_url] => [skupina_www] => Array ( ) [url] => /research-groups/cibulka-en/people [sablona] => stdClass Object ( [class] => stranka [html] => [css] => [js] => [autonomni] => 1 ) ) [38632] => stdClass Object ( [nazev] => [seo_title] => Research [seo_desc] => [autor] => [autor_email] => [obsah] =>Tuning the Photophysical Properties of Flavins by Attaching an Aryl Moiety via Direct C–C Bond Coupling
Abstract: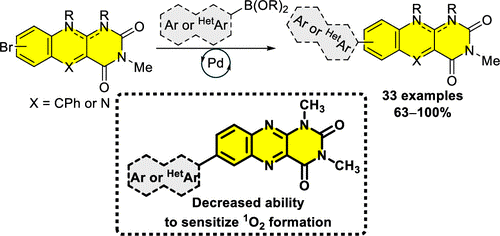 Palladium-catalyzed Suzuki reactions of brominated flavin derivatives (5-deazaflavins, alloxazines, and isoalloxazines) with boronic acids or boronic acid esters that occur readily under mild conditions were shown to be an effective tool for the synthesis of a broad range of 7/8-arylflavins. In general, the introduction of an aryl/heteroaryl group by means of a direct C–C bond has been shown to be a promising approach to tuning the photophysical properties of flavin derivatives. The aryl substituents caused a bathochromic shift in the absorption spectra of up to 52 nm and prolonged the fluorescence lifetime by up to 1 order of magnitude. Moreover, arylation of flavin derivatives decreased their ability to generate singlet oxygen.
Palladium-catalyzed Suzuki reactions of brominated flavin derivatives (5-deazaflavins, alloxazines, and isoalloxazines) with boronic acids or boronic acid esters that occur readily under mild conditions were shown to be an effective tool for the synthesis of a broad range of 7/8-arylflavins. In general, the introduction of an aryl/heteroaryl group by means of a direct C–C bond has been shown to be a promising approach to tuning the photophysical properties of flavin derivatives. The aryl substituents caused a bathochromic shift in the absorption spectra of up to 52 nm and prolonged the fluorescence lifetime by up to 1 order of magnitude. Moreover, arylation of flavin derivatives decreased their ability to generate singlet oxygen.
Highly Chemoselective Catalytic Photooxidations by Using Solvent as a Sacrificial Electron Acceptor
Abstract:
Catalyst recovery is an integral part of photoredox catalysis. It is often solved by adding another component-a sacrificial agent-whose role is to convert the catalyst back into its original oxidation state. However, an additive may cause a side reaction thus decreasing the selectivity and overall efficiency. Herein, we present a novel approach towards chemoselective photooxidation reactions based on suitable solvent-acetonitrile acting simultaneously as an electron acceptor for catalyst recovery, and on anaerobic conditions. This is allowed by the unique properties of the catalyst, 7,8-dimethoxy-3-methyl-5-phenyl-5-deazaflavinium chloride existing in both strongly oxidizing and reducing forms, whose strength is increased by excitation with visible light. Usefulness of this system is demonstrated in chemoselective dehydrogenations of 4-methoxy- and 4-chlorobenzyl alcohols to aldehydes without over-oxidation to benzoic acids achieving yields up to 70 %. 4-Substituted 1-phenylethanols were oxidized to ketones with yields 80–100 % and, moreover, with yields 31-98 % in the presence of benzylic methyl group, diphenylmethane or thioanisole which are readily oxidized in the presence of oxygen but these were untouched with our system. Mechanistic studies based on UV-Vis spectro-electrochemistry, EPR and time-resolved spectroscopy measurements showed that the process involving an electron release from an excited deazaflavin radical to acetonitrile under formation of solvated electron is crucial for the catalyst recovery.
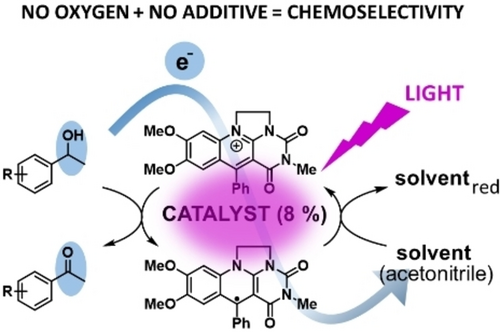 |
New way to drive photoredox catalysis: Highly chemoselective photooxidations of benzylic alcohols to carbonyl compounds in the presence of various easily-oxidizable groups are possible in a simple oxygen-free system consisting of a substrate, unique deazaflavinium catalyst and acetonitrile which acts simultaneously as a sacrificial electron acceptor and solvent. |
Tuning Deazaflavins Towards Highly Potent Reducing Photocatalysts Guided by Mechanistic Understanding – Enhancement of the Key Step by the Internal Heavy Atom Effect
Abstract:
Deazaflavins are well suited for reductive chemistry acting via a consecutive photo-induced electron transfer, in which their triplet state and semiquinone – the latter is formed from the former after electron transfer from a sacrificial electron donor – are key intermediates. Guided by mechanistic investigations aiming to increase intersystem crossing by the internal heavy atom effect and optimising the concentration conditions to avoid unproductive excited singlet reactions, we synthesised 5-aryldeazaflavins with Br or Cl substituents on different structural positions via a three-component reaction. Bromination of the deazaisoalloxazine core leads to almost 100 % triplet yield but causes photo-instability and enhances unproductive side reactions. Bromine on the 5-phenyl group in ortho position does not affect the photostability, increases the triplet yield, and allows its efficient usage in the photocatalytic dehalogenation of bromo- and chloroarenes with electron-donating methoxy and alkyl groups even under aerobic conditions. Reductive powers comparable to lithium are achieved.
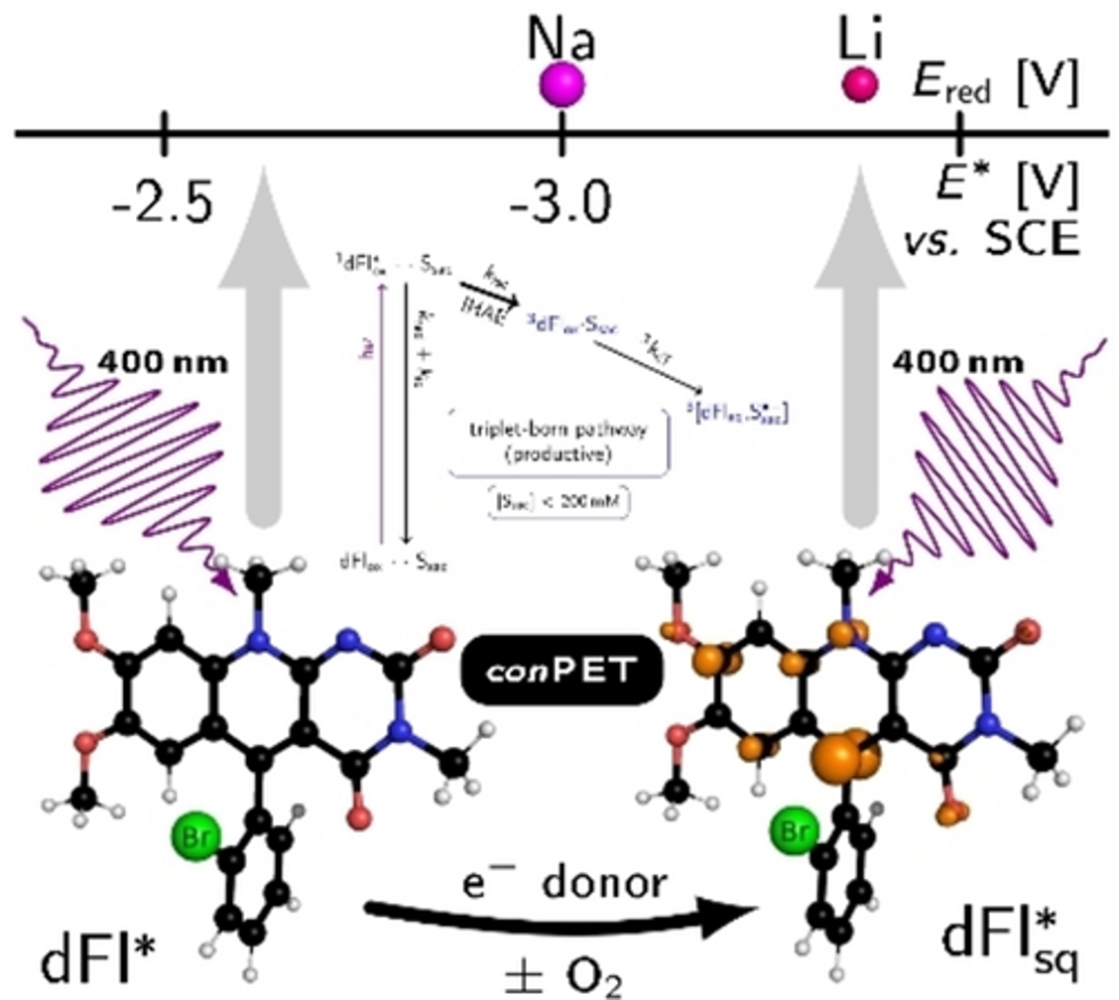 |
Inspired by nature and based on mechanistic understanding, we designed deazaflavins (dFl) with reducing powers comparable to Li which function via the consecutive photo-induced electron transfer (conPET) mechanism independently on molecular oxygen. The internal heavy atom effect (IHAE) by introducing bromine into the photocatalyst enhances considerably the key triplet pathway via a triplet-born radical pair. Furthermore, an optimal concentration of the sacrificial electron donor (Ssac) is required for bypassing the unproductive reaction via the singlet-born radical pair. |
Photophysical properties of alloxazine derivatives with extended aromaticity – Potential redox-sensitive fluorescent probe
DOI: 10.1016/j.saa.2022.120985
Abstract: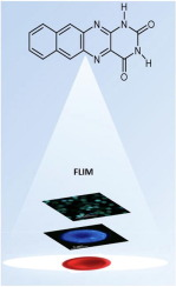 The spectral and photophysical properties of two four-ring alloxazine derivatives, naphtho[2,3-g]pteridine-2,4(1H,3H)-dione (1a) and 1,3-dimethylnaphtho[2,3-g]pteridine-2,4(1H,3H)-dione, (1b) were studied. The propensity of 1a for excited-state proton transfer reactions in the presence of acetic acid as a catalyst was also studied, showing no signature of the reaction occurring. In addition, quenching of 1a fluorescence by acetic acid was investigated. Singlet and triplet states and spectral data for 1a and 1b were calculated using density functional theory TD-DFT at B3LYP/6-31G(d) and UB3LYP levels. Finally, fluorescence lifetime imaging microscopy (FLIM) using 1a and 1b as fluorescence probes was applied to in vitro human red blood cells (RBCs) with and without tert-butyl hydroperoxide (TB) as an oxidising agent. To evaluate and compare the effects of 1a and 1b on the redox properties of RBCs, the fluorescence lifetime, amplitude and fractional intensities were calculated, and phasor plot analysis was performed. The results obtained show the appearance of a new proximal cluster in the phasor fingerprint of RBCs in the presence of 1b and a shorter fluorescence lifetime of RBCs in the presence of 1a.
The spectral and photophysical properties of two four-ring alloxazine derivatives, naphtho[2,3-g]pteridine-2,4(1H,3H)-dione (1a) and 1,3-dimethylnaphtho[2,3-g]pteridine-2,4(1H,3H)-dione, (1b) were studied. The propensity of 1a for excited-state proton transfer reactions in the presence of acetic acid as a catalyst was also studied, showing no signature of the reaction occurring. In addition, quenching of 1a fluorescence by acetic acid was investigated. Singlet and triplet states and spectral data for 1a and 1b were calculated using density functional theory TD-DFT at B3LYP/6-31G(d) and UB3LYP levels. Finally, fluorescence lifetime imaging microscopy (FLIM) using 1a and 1b as fluorescence probes was applied to in vitro human red blood cells (RBCs) with and without tert-butyl hydroperoxide (TB) as an oxidising agent. To evaluate and compare the effects of 1a and 1b on the redox properties of RBCs, the fluorescence lifetime, amplitude and fractional intensities were calculated, and phasor plot analysis was performed. The results obtained show the appearance of a new proximal cluster in the phasor fingerprint of RBCs in the presence of 1b and a shorter fluorescence lifetime of RBCs in the presence of 1a.
Amide Bond Formation via Aerobic Photooxidative Coupling of Aldehydes with Amines Catalyzed by a Riboflavin Derivative
doi.org/10.1021/acs.orglett.1c02391
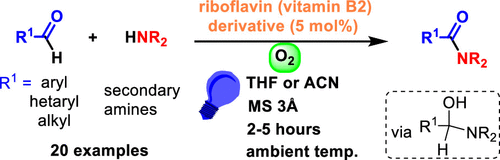 Abstract:
Abstract:
We report an effective, operationally simple, and environmentally friendly system for the synthesis of tertiary amides by the oxidative coupling of aromatic or aliphatic aldehydes with amines mediated by riboflavin tetraacetate (RFTA), an inexpensive organic photocatalyst, and visible light using oxygen as the sole oxidant. The method is based on the oxidative power of an excited flavin catalyst and the relatively low oxidation potential of the hemiaminal formed by amine to aldehyde addition.
Flavin-Helicene Amphiphilic Hybrids: Synthesis, Characterization, and Preparation of Surface-Supported Films
doi.org/10.1002/cplu.202100092
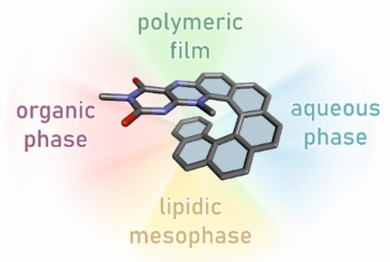 The complex characterization of a flavo[7]helicene conjugate is reported in this work. This conjugate combines inherent helical chirality with redox activity, which was studied in solution (both aqueous and organic phases), in layers (electropolymers), and in the solid state (a single crystal).
The complex characterization of a flavo[7]helicene conjugate is reported in this work. This conjugate combines inherent helical chirality with redox activity, which was studied in solution (both aqueous and organic phases), in layers (electropolymers), and in the solid state (a single crystal).
Abstract:
This work reports on the preparation and structural characterization of flavo[7]helicene 1 (flavin-[7]helicene conjugate), which was subsequently characterized at the molecular level in either an aqueous environment or an organic phase, at the supramolecular level in the form of polymeric layers, and also embedded in a lipidic mesophase environment to study the resulting properties of such a hybrid relative to its parent molecules. The flavin benzo[g]pteridin-2,4-dione (isoalloxazine) was selected for conjugation because of its photoactivity and reversible redox behavior. Compound 1 was prepared from 2-nitroso[6]helicene and 6-methylamino-3-methyluracil, and characterized using common structural and spectroscopic tools: circular dichroism (CD), circularly polarized luminescence (CPL) spectroscopy, cyclic voltammetry (CV), and DFT quantum calculations. In addition, a methodology that allows the loading of 1 enantiomers into an internally nanostructured lipid (1-monoolein) matrix was developed.
Robust Photocatalytic Method Using Ethylene-Bridged Flavinium Salts for the Aerobic Oxidation of Unactivated Benzylic Substrates
doi.org/10.1002/adsc.202100024
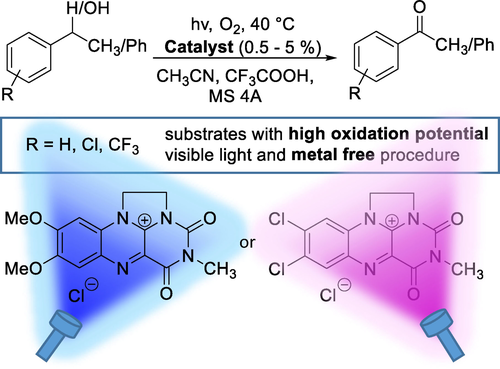 Abstract:
Abstract:
7,8-Dimethoxy-3-methyl-1,10-ethylenealloxazinium chloride (1a) was found to be a superior photooxidation catalyst among substituted ethylene-bridged flavinium salts (R=7,8-diMeO, 7,8-OCH2O-, 7,8-diMe, H, 7,8-diCl, 7-CF3 and 8-CF3). Selection was carried out based on structure vs catalytic activity and properties relationship investigations. Flavinium salt 1a proved to be robust enough for practical applications in benzylic oxidations/oxygenations, which was demonstrated using a series of substrates with high oxidation potential, i. e., 1-phenylethanol, ethylbenzene, diphenylmethane and diphenylmethanol derivatives substituted with electron-withdrawing groups (Cl or CF3). The unique capabilities of 1a can be attributed to its high photostability and participation via a relatively long-lived singlet excited state, which was confirmed using spectroscopic studies, electrochemical measurements and TD-DFT calculations. This allows the maximum use of the oxidation power of 1a, which is given by its singlet excited state reduction potential of +2.4 V. 7,8-Dichloro-3-methyl-1,10-ethylenealloxazinium chloride (1 h) can be used as an alternative photocatalyst for even more difficult substrates.
Photocatalytic Oxidative [2+2] Cycloelimination Reactions with Flavinium Salts: Mechanistic Study and Influence of the Catalyst Structure
doi.org/10.1002/cplu.202000767
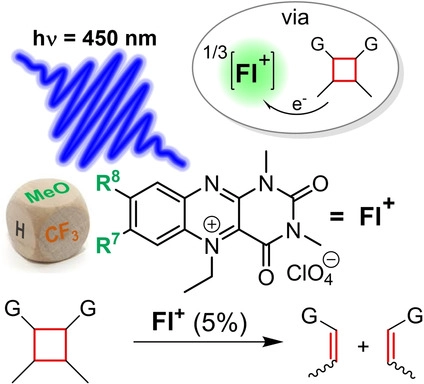
Making light work: Alloxazinium salts (flavin derivatives) are very strong oxidizing agents in their excited singlet and triplet states after absorption of visible light. When suitably substituted, these salts are relatively photostable and robust for practical applications in photoredox catalysis, as shown by the detailed mechanistic study on the [2+2] photocycloelimination reaction mediated by 7,8-dimethoxy-1,3-dimethylalloxazinium perchlorate.
Abstract:
Flavinium salts are frequently used in organocatalysis but their application in photoredox catalysis has not been systematically investigated to date. We synthesized a series of 5-ethyl-1,3-dimethylalloxazinium salts with different substituents in the positions 7 and 8 and investigated their application in light-dependent oxidative cycloelimination of cyclobutanes. Detailed mechanistic investigations with a coumarin dimer as a model substrate reveal that the reaction preferentially occurs via the triplet-born radical pair after electron transfer from the substrate to the triplet state of an alloxazinium salt. The very photostable 7,8-dimethoxy derivative is a superior catalyst with a sufficiently high oxidation power (E*=2.26 V) allowing the conversion of various cyclobutanes (with Eox up to 2.05 V) in high yields. Even compounds such as all-trans dimethyl 3,4-bis(4-methoxyphenyl)cyclobutane-1,2-dicarboxylate can be converted, whose opening requires a high activation energy due to a missing pre-activation caused by bulky adjacent substituents in cis-position.
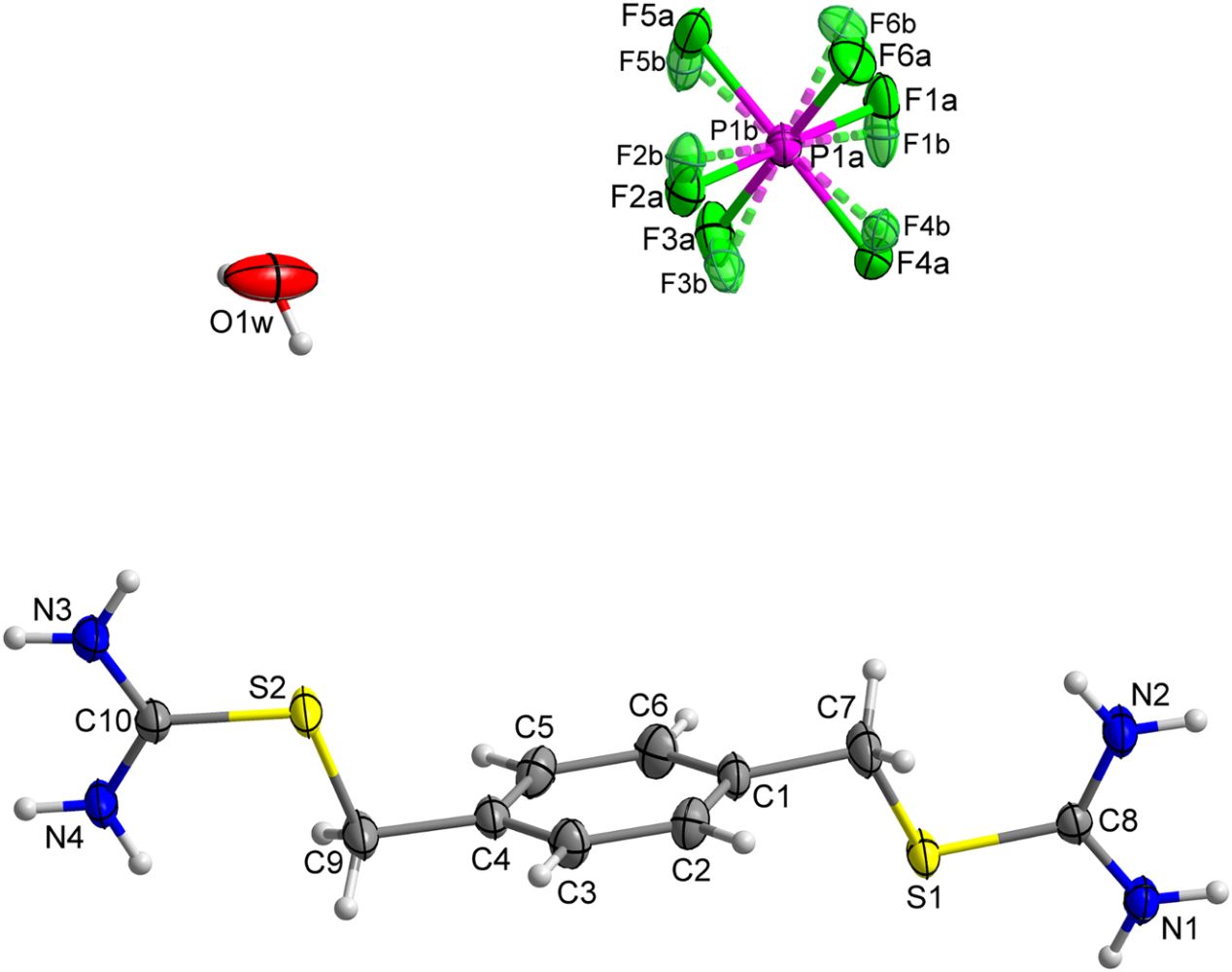
The crystal structure of 2-(4-((carbamimidoylthio)methyl)-benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
doi.org/10.1515/ncrs-2021-0417
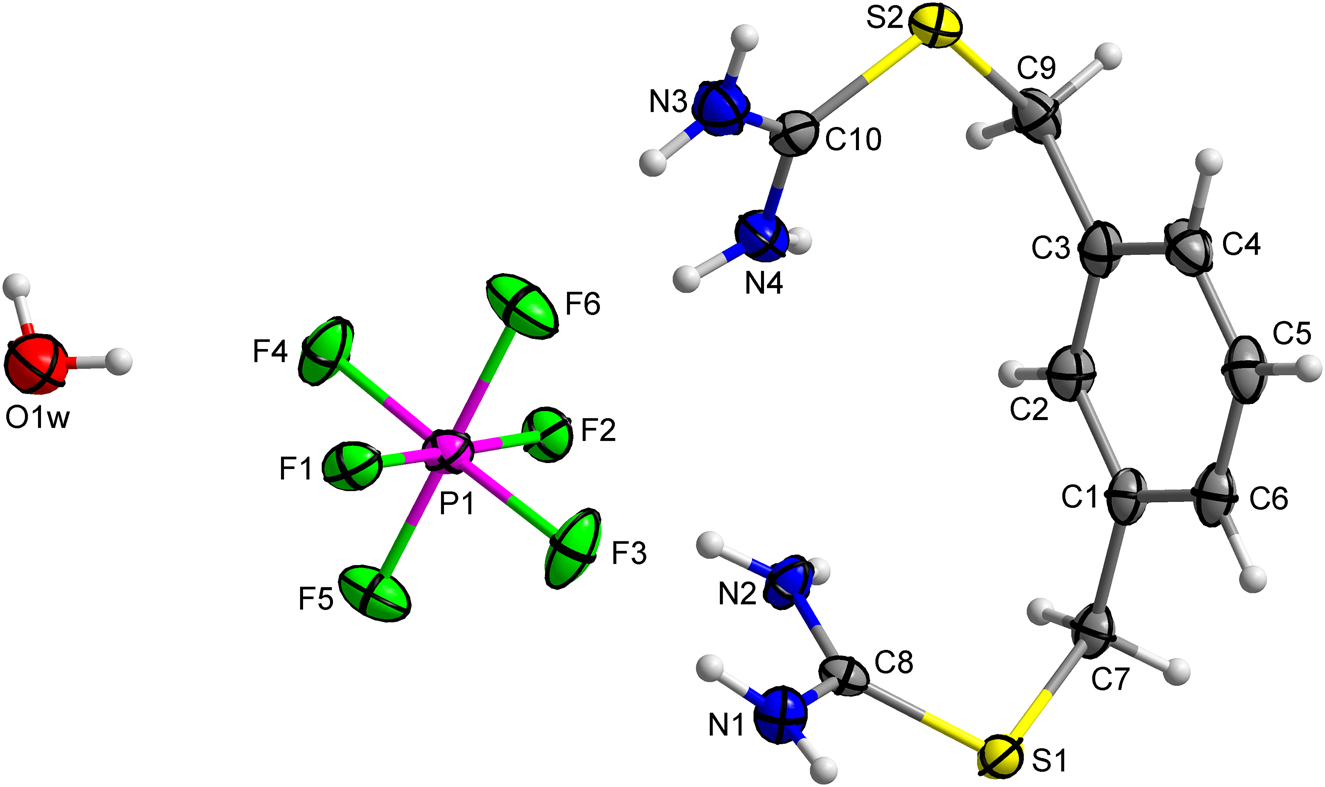
Crystal structure of 2-(3-((carbamimidoylthio)methyl)-benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
doi.org/10.1515/ncrs-2021-0418
2024
Weisheitelová I., Varma N., Chudoba J., Burdziński G., Sikorski M., Cibulka R.: Catalyst-free aerobic photooxidation of sensitive benzylic alcohols with chemoselectivity controlled by DMSO solvent. Green Chem. 2024 accepted. DOI:10.1039/D4GC00087K
Burešová Z., Gobeze H. B., Grygarová M., Pytela O., Klikar M., Obertík R., Cibulka R., Islam T., Schanze K. S., Bureš F.: Dicyanopyrazine Photoredox Catalysts: Correlation of Efficiency with Photophysics and Electronic Structure. Journal of Catalysis 2024, 430, 115348. DOI:10.1016/j.jcat.2024.115348
Zubová E., Pokluda A. Dvořáková H., Krupička M., Cibulka R.: Exploring the Reactivity of Flavins with Nucleophiles Using a Theoretical and Experimental Approach. ChemPlusChem 2024, e202300547. DOI:10.1002/cplu.202300547.
2023
Pavlovska T., Weisheitelová I., Pramthaisong C., Sikorski M., Jahn U., Cibulka R.: Primary and Secondary Amines by Flavin‐Photocatalyzed Consecutive Desulfonylation and Dealkylation of Sulfonamides. Adv. Synth. Catal. 2023, 365, 4662. DOI:10.1002/adsc.202300843.
Golczak A., Prukała D., Gierszewski M., Cherkas V., Kwiatek D., Kubiak A., Varma N., Pedziński T., Murphree S., Cibulka R., Mrówczińska L., Kolanovski J. L., Sikorski M.: Tetramethylalloxazines as efficient singlet oxygen photosensitizers and potential redox‑sensitive agents. Scientific Reports 2023, 13, 13426. DOI:10.1038/s41598-023-40536-4.
Insińska-Rak M., Golczak A., Gierszewski M., Anwar Z., Cherkas V., Kwiatek D., Sikorska E., Khmelinskii I., Burdziński G., Cibulka R., Mrówczyńskag L., Kolanowskic J. L. and Sikorski M.: 5-Deazaalloxazine as photosensitizer of singlet oxygen and potential redox-sensitive agent. Photochem. Photobiol. Sci. 2023. DOI:10.1007/s43630-023-00401-9
Pokluda A., Zubova E., Chudoba J., Krupička M., Cibulka R.: Catalytic Artificial Nitroalkane Oxidase – a Way Towards Organocatalytic Umpolung. Org. Biomol. Chem. 2023, 21, 2768 - 2774. DOI:10.1039/d3ob00101f.
2022
Čubiňák M., Varma N., Oeser P., Pokluda A., Pavlovska T., Cibulka R., Sikorski M., Tobrman T. Tuning the Photophysical Properties of Flavins by Attaching an Aryl Moiety via Direct C–C Bond Coupling. J. Org. Chem. 2022. DOI:10.1021/acs.joc.2c02168
Obertík R., Chudoba J., Šturala J., Tarábek J., Ludvíková L., Slanina T., König B., Cibulka R.: Chem. Eur. J. 2022, accepted as VIP. DOI:10.1002/chem.202202487.
Pavlovska T., Král Lesný D., Svobodová E., Hoskovcová I., Archipowa N., Kutta R. J., Cibulka R. Tuning Deazaflavins Towards Highly Potent Reducing Photocatalysts Guided by Mechanistic Understanding - Enhancement of the Key Step by the Internal Heavy Atom Effect. Chem. Eur. J. 2022, 28, e20220076. Hot Paper. DOI:10.1002/chem.202200768.
Golczak A., Insińska-Rak M., Davoudpour A., Saeed D. H., Ménová P., Mojr V., Cibulka R., Khmelinskii V., Mrówczyńska L., Sikorski M. Photophysical properties of alloxazine derivatives with extended aromaticity – potential redox sensitive fluorescent probe. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, volume 272, 120985, 2022. DOI: 10.1016/j.saa.2022.120985.
2021
Tolba A. H., Krupička M., Chudoba J., Cibulka R.: Amide bond formation via aerobic photooxidative coupling of alde-hydes with amines catalyzed by a riboflavin derivative Org. Lett. 2021, 23, 17, 6825–6830. DOI:10.1021/acs.orglett.1c02391.
Radek Cibulka (a prof. M. Fraaije z Univerzity v Groningenu) editory knihy „Flavin-Based catalysis“ v nakladatelství Wiley-VCH. Zároveň členové naší skupiny připravili tři z dvanácti kapitol v této knize:
Pavlovska, T.; Cibulka, R., Structure and Properties of Flavins. pp 1-27.
Cibulka, R.; Fraaije, M. W., Modes of Flavin-Based Catalysis. pp 97-124.
Svobodová, E.; Cibulka, R., New Applications of Flavin Photocatalysis. pp 265-291.
Wiley-VCH - Flavin-Based Catalysis
Jakubec M., Novák D., Zatloukalová M., Sýkora J., Císařová I., Cibulka R., Favereau L., Crassous J., Bilewicz R., Hrbáč J., Storch J., Žádný J., Vacek J.: Flavin-Helicene Amphiphilic Hybrids: Synthesis, Characterization, and Preparation of Surface-Supported Films ChemPlusChem 2021, 86, 982-990. DOI: 10.1002/cplu.202100092.
Pokluda A., Anwar Z., Boguschová V., Anusiewicz I., Skurski P., Sikorski M., Cibulka R.: Robust photocatalytic method using ethylene-bridged flavinium salts for the aerobic oxidation of unactivated benzylic substrates Adv. Synth. Catal. 2021 363, 4371-4379. VIP, DOI: 10.1002/adsc.202100024.
Hartman T., Reisnerová M., Chudoba J., Svobodová E., Archipowa N., Kutta R. J., Cibulka R.: Photocatalytic Oxidative [2+2] Cycloelimination Reactions with Flavinium Salts: Mechanistic Study and Influence of the Catalyst Structure ChemPlusChem 2021, 86, 373–386. DOI: 10.1002/cplu.202000767.
Eigner, Václav and Holakovský, Roman. "The crystal structure of 2-(4-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2" Zeitschrift für Kristallographie - New Crystal Structures, vol. , no. , 2021. doi.org/10.1515/ncrs-2021-0417
Eigner, Václav and Holakovský, Roman. "Crystal structure of 2-(3-((carbamimidoylthio)methyl)benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2 " Zeitschrift für Kristallographie - New Crystal Structures, vol. , no. , 2021. doi.org/10.1515/ncrs-2021-0418
2020
Graml A., Neveselý T., Kutta R.-J., Cibulka R., König B.: Deazaflavin reductive photocatalysis involves excited semiquinone radicals Nature Comm. 2020, 11, 3174. DOI: 10.1038/s41467-020-16909-y. https://rdcu.be/b47PV
Cibulka R.: Strong chemical reducing agents produced by light Nature 580, 31-32 (2020). DOI: 10.1038/d41586-020-00872-1. https://www.nature.com/articles/d41586-020-00872-1
Tolba A. H., Vávra F., Chudoba J., Cibulka R: Tuning flavin-based photocatalytic systems for application in the mild chemoselective aerobic oxidation of benzylic substrates Eur. J. Org. Chem. 2020, 1579–1585. DOI:10.1002/ejoc.201901628. Special issue Photochemical synthesis.
Schlosser J., Cibulka R., Groß P., Ihmels H., Mohrschladt C. J.: Visible-light induced di-π-methane rearrangement of dibenzobarrelene derivatives ChemPhotoChem 2020, 4, 132. DOI:10.1002/cptc.201900221.
2019
Zelenka, J.; Cibulka, R., Roithová, J.: Flavinium catalyzed photooxidation: Detection and characterization of elusive peroxyflavinium intermediates. Angew. Chem. Int. Ed. 2019, 58, 15412-15420. DOI:10.1002/anie.201906293. VIP

März M., Babor M., Cibulka R.: Flavin Catalysis Employing an N(5)-Adduct: An Application in the Aerobic Organocatalytic Mitsunobu Reaction Eur. J. Org. Chem. 2019, 20, 3264-3268. DOI:10.1002/ejoc.201900397.
Pokluda A., Kohout M., Chudoba J., Krupička M., Cibulka R.: Nitrosobenzene – Reagent for the Mitsunobu Esterification Reaction ACS Omega 2019, 4, 5012-5018. DOI:10.1021/acsomega.8b03551.
Valenta L., Kovaříček P., Valeš V., Bastl Z., Drogowska K. A., Verhagen T. A., Cibulka R., Kalbáč M.: Spatially resolved covalent functionalization patterns on graphene Angew. Chem. Int. Ed. 2019, 58, 1324-1328. DOI:10.1002/anie.201810119.
https://onlinelibrary.wiley.com/doi/pdf/10.1002/anie.201810119
Zelenka J., Svobodová E., Tarábek J., Hoskovcová I., Boguschová V., Bailly S., Sikorski M., Roithová J., Cibulka R.: Combining flavin photocatalysis and organocatalysis: metal-free aerobic oxidation of unactivated benzylic substrates Org. Lett. 2019, 21, 114-119. DOI:10.1021/acs.orglett.8b03547. Highly cited paper (WOS).
2018
März M., Kohout M., Neveselý T., Chudoba J., Prukala D., Niziński S., Sikorski M., Burdziński G., Cibulka R.: Azodicarboxylate-free esterification with triphenylphosphine mediated by flavin and visible light: method development and stereoselectivity control Org. Biomol. Chem. 2018, 16, 6809-6817. DOI: 10.1039/C8OB01822G.
Holakovský R., Cibulka R., Konečková E., März M.: Způsob stanovení enantiomerní čistoty chirálních sulfoxidů pomocí 1H NMR analýzy, PV 2016-92, č. 307419. Pozn. 2018
König B., Kümmel S., Svobodová E. and Cibulka R.: Flavin photocatalysis, in Physical Sciences Reviews, 3 (8), 20170168, ISSN (Online) 2365-659X. DOI: 10.1515/psr-2017-0168.
Slavíček P., Cibulka R.: A chemici nebudou mít co žrát… (Strojové učení v chemii) Vesmír 97, 2018 (5), 300-301.
https://vesmir.cz/cz/casopis/archiv-casopisu/2018/cislo-5/a-chemici-nebudou-mit-co-zrat.html
Muchová E., Bezek M., Suchan J., Cibulka R., Slavíček P.: Molecular Dynamics and Metadynamics Simulations of [2+2] Photocycloaddition Int. J. Quantum Chem. 2018, 118, e25534. DOI:10.1002/qua.25534
Cuřínová P., Dračínský M., Jakubec M, Tlustý M., Janků K., Izák P., Holakovský R.: Enantioselective complexation of 1‐phenylethanol with chiral compounds bearing urea moiety. Chirality 2018, 30, 798–806. DOI:10.1002/chir.22855.
Mojr V., Pitrová G., Straková K., Prukala D., Brazevic S., Svobodová E., Hoskovcová I., Burdziński G., Slanina T., Sikorski M., Cibulka R.: Flavin Photocatalysts for visible Light [2+2] Cycloadditions: Structure, Reactivity and Reaction Mechanism ChemCatChem 2018, 10, 849-858. DOI:10.1002/cctc.201701490.
Kurfiřt M., Špačková J., Svobodová E., Cibulka R.: Flavin derivatives immobilized on mesoporous silica: a versatile tool in visible-light photooxidation reactions Monatshefte Chem. 2018, 149, 863-869. DOI:10.1007/s00706-017-2127-1.
2017
Žurek J., Svobodová E., Šturala J., Dvořáková H., Svoboda J., Cibulka R.: Chiral ethylene-bridged flavinium salts: The stereoselectivity of flavin-10a-hydroperoxide formation and the effect of substitution on the photochemical properties Tetrahedron Asymmetry 2017, 28, 1780-1791, DOI:10.1016/j.tetasy.2017.10.029
Sofer, Z., Luxa, J., Bouša, D., Sedmidubský, D., Lazar, P., Hartman, T., Hardtdegen, H. and Pumera, M.: The Covalent Functionalization of Layered Black Phosphorus by Nucleophilic Reagents. Angew. Chem. Int. Ed. 2017, 56, 9891–9896. DOI:10.1002/anie.201705722
März M., Kohout M., Chudoba J., Cibulka R.: Photocatalytic Esterification under Mitsunobu Reaction Conditions Mediated by Flavin and Visible Light Org. Biomol. Chem. 2017, 15, 1970-1975. DOI:10.1039/C6OB02770A, highlighted in http://www.organic-chemistry.org/Highlights/2018/12March.shtm
Kolderová, N.; Neveselý, T.; Šturala, J.; Kuchař, M.; Holakovský, R.; Kohout, M.: Enantioseparation of Chiral Sulfoxides on Amylose-Based Columns: Comparison of Normal Phase Liquid Chromatography and Supercritical Fluid Chromatography. Chromatographia 2017, 80, 547-557. DOI: 10.1007/s10337-016-3234-6
Špačková J., Svobodová E., Hartman T., Stibor I., Kopecká J., Cibulková J., Chudoba J., Cibulka R.: Visible Light [2+2] Photocycloaddition Mediated by Flavin Derivative Immobilized on Mesoporous Silica, ChemCatChem 2017, 9, 1177-1181. DOI:10.1002/cctc.201601654
Jirásek M., Straková K., Neveselý T., Svobodová E., Rottnerová Z., Cibulka R.: Flavin-Mediated Visible Light [2+2] Photocycloadditon of Nitrogen and Sulfur-Containing Dienes, Eur. J. Org. Chem. 2017, 2139-2146. DOI:10.1002/ejoc.201601377
2016
Hartman T., Cibulka R.: Photocatalytic Systems with Flavinium Salts: From Photolyase Models to Synthetic Tool for Cyclobutane Ring Opening, Org. Lett. 2016, 18, 3710-3713. DOI:10.1021/acs.orglett.6b01743
Bím, D.; Svobodová, E.; Eigner, V.; Rulíšek, L.; Hodačová, J.: Copper(II) and Zinc(II) Complexes of Conformationally Constrained Polyazamacrocycles as Efficient Catalysts for RNA Model Substrate Cleavage in Aqueous Solution at Physiological pH, Chem. Eur. J. 2016, 22, 10426-10437. DOI:10.1002/chem.201683062
Neveselý T., Svobodová E., Chudoba J., Sikorski M., Cibulka R.: Efficient metal-free aerobic photooxidation of sulfides to sulfoxides mediated by a vitamin B2 derivative and visible light, Adv. Synth. Catal. 2016, 358, 1654–1663. DOI:10.1002/adsc.201501123 VIP.
Holakovský R., Cibulka R., Konečková E., März M.: Močovina na bázi binaftalenu pro stanovení enantiomerní čistoty chirálních sulfoxidů pomocí 1H-NMR analýzy. Patent č. 305 940 (PV: 00710-14)
2015
García, Y. R.; Zelenka, J.; Pabon, Y. V.; Iyer, A.; Buděšínský, M.; Kraus, T.; Smith, C. I. E.; Madder, A.: Cyclodextrin–peptide conjugates for sequence specific DNA binding, Org. Biomol. Chem. 2015, 13, 5273–5278. DOI:10.1039/C5OB00609K
Bousa, D.; Jankovsky, O.; Sedmidubsky, D.; Luxa, J.; Sturala, J.; Pumera, M.; Sofer, Z.: Mesomeric Effects of Graphene Modified with Diazonium Salts: Substituent Type and Position Influence its Properties, Chem. Eur. J. 2015, 21, 17728-17738. DOI:10.1002/chem.201502127
Tomanová P., Šturala J., Buděšínský M., Cibulka R.: A click chemistry approach towards flavin-cyclodextrin conjugates – bioinspired sulfoxidation catalysts, Molecules 2015, 20, 19837-19848. DOI:10.3390/molecules201119667
Holakovský R., März M., Cibulka R.: Urea derivatives based on a 1,1'-binaphthalene skeleton as chiral solvating agents for sulfoxides, Tetrahedron: Asymmetry 2015, 26, 1328-1334. DOI:10.1016/j.tetasy.2015.10.011
Hartman J., Šturala J., Cibulka R.: Two-phase Oxidations with Aqueous Hydrogen Peroxide Catalysed by Amphiphilic Pyridinium and Diazinium Salts, Adv. Synth. Catal. 2015, 357, 3573-3586. DOI:10.1002/adsc.201500687
Mojr V., Svobodová E., Straková K., Neveselý T., Chudoba J., Dvořáková H., Cibulka R.: Tailoring Flavins for Visible Light Photocatalysis: Organocatalytic [2+2] Cycloadditions Mediated by a Flavin Derivative and Visible Light, Chem. Comm. 2015, 51, 12036 – 12039. DOI:10.1039/C5CC01344E
Sofer, Z.; Jankovský, O.; Šimek, P.; Sedmidubský, D.; Šturala, J.; Kosina, J.; Mikšová, R.; Macková, A.; Mikulics, M.; Pumera, M.: Insight into the Mechanism of the Thermal Reduction of Graphite Oxide: Deuterium-Labeled Graphite Oxide Is the Key, ACS Nano; 2015, 9, 5478-5485. DOI:10.1021/acsnano.5b01463
Šturala J., Boháčová S., Chudoba J., Metelková R., Cibulka R.: Electron-deficient Heteroarenium salts – an Organocatalytic Tool for Activation of Hydrogen Peroxide in Oxidations, J. Org. Chem., 2015, 80, 2676-2699. DOI:10.1021/jo502865f
Cibulka R.: Artificial flavin systems for chemoselective and stereoselective oxidations (Microreview), Eur. J. Org. Chem., 2015, 915-932. DOI:10.1002/ejoc.201403275 + Cover picture
2014
Zelenka J., Hartman T. Klímová K., Hampl F., Cibulka R.: Phase-transfer catalysis in oxidations based on the covalent bonding of hydrogen peroxide to amphiphilic flavinium salts, ChemCatChem, 2014, 6, 2843-2846. DOI:10.1002/cctc.201402533
Kotoučová H., Strnadová I., Kovandová M., Chudoba J., Dvořáková H., Cibulka R.: Biomimetic aerobic oxidative hydroxylation of arylboronic acids to phenols catalysed by a flavin derivative, Org. Biomol. Chem., 2014, 12, 2137-2142. DOI:10.1039/C3OB42081G
2013
Ménová P., Dvořáková H., Eigner V. Ludvík J., Cibulka R.: Electron-deficient alloxazinium salts: efficient organocatalysts of mild and chemoselective sulfoxidations with hydrogen peroxide, Adv. Synth. Catal., 2013, 355, 3451-3462. DOI:10.1002/adsc.201300617
Kümmel S., Cibulka R., König B.: “Flavin Photocatalysis” in Chemical Photocatalysis (Burkhard König, ed.), de Gruyter, Berlin 2013, ISBN: 978-3-11-026924-6.
Jurok R., Hodačová J., Eigner V., Dvořáková H., Setnička V., Cibulka R.: Planar chiral flavinium salts: Synthesis and evaluation of the effect of substituents on the catalytic efficiency in enantioselective sulfoxidation reactions, Eur. J. Org. Chem., 2013, 7724-7738. DOI:10.1002/ejoc.201300847
Daďová J., Kümmel S., Feldmeier C., Cibulková J., Pažout R., Maixner J., Gschwind R. M., König B., Cibulka R.: Aggregation effects in visible light flavin photocatalysts: Synthesis, structure and catalytic activity of 10-arylflavins, Chem. Eur. J., 2013, 19, 1066-1075. DOI:10.1002/chem.201202488
2012
Hartman T., Herzig V., Buděšínský M., Jindřich J., Cibulka R., Kraus T.: Flavin-cyclodextrin conjugates: effect of the structure on catalytic activity in enantioselective sulfoxidations, Tetrahedron: Asymmetry, 2012, 23, 1571-1583. DOI:10.1016/j.tetasy.2012.10.017
Šturala J., Cibulka R.: Novel synthesis of symmetrical dinitro and diamino Tröger’s base analogues, Eur. J. Org. Chem., 2012, 7066-7074. DOI:10.1002/ejoc.201201188
Cibulka R., Jurok R.: ORGANOKATALYTICKÉ ENANTIOSELEKTIVNÍ OXIDACE SULFIDŮ NA SULFOXIDY, Chemické listy, 2012, 106, 896-902. http://www.chemicke-listy.cz/docs/full/2012_10_896-902.pdf
Ménová P., Cibulka R.: INSIGHT INTO THE CATALYTIC ACTIVITY OF ALLOXAZINIUM AND ISOALLOXAZINIUM SALTS IN THE OXIDATIONS OF SULFIDES AND AMINES WITH HYDROGEN PEROXIDE, J. Mol. Catal. A: Chemical, 2012, 363-364, 362-370. DOI:10.1016/j.molcata.2012.07.012
Daďová J., Svobodová E., Sikorski M., König B., Cibulka R.: Photooxidation of sulfides to sulfoxides mediated by tetra-O-acetylriboflavin and visible light. ChemCatChem, 2012, 4, 620-623. DOI:10.1002/cctc.201100372
Tisková zpráva:"Blue Light District: Sulfide Photooxidation" (ChemistryViews.org)
2011
Mojr V., Budesinsky M., Cibulka R., Kraus T.: Alloxazine-cyclodextrin conjugates for organocatalytic enantioselective sulfoxidations. Org. Biomol. Chem., 2011, 9, 7257-7580. DOI:10.1039/C1OB05934C + Cover picture
Ménová P., Eigner V., Čejka J., Dvořáková H., Šanda M., Cibulka R.: Synthesis and structural studies of flavin and alloxazine adducts with O-nucleophiles. J. Mol. Struct. 2011, 1004, 178-187. DOI:10.1016/j.molstruc.2011.08.002
Sayin S., Akkus G. U., Cibulka R., Stibor I., Yilmaz M: Synthesis of Flavin-Calix[4]arene Conjugate Derivatives. Helv. Chim. Acta 2011, 94, 481-485. DOI:10.1002/hlca.201000260
Ménová P., Kafka F., Dvořáková H., Gunnoo S., Šanda M., Cibulka R.: Pyrazinium Salts as Efficient Organocatalysts of Mild Oxidations with Hydrogen Peroxide. Adv. Synth. Catal. 2011, 353, 865-870. DOI:10.1002/adsc.201000906
2010
Rohlíček J. Cibulka R., Cibulková J., Maixner J., Hušák M.: 10-Methylisoalloxazine-5-oxide from synchrotron powder diffraction data. Acta Cryst. 2010, E66, o3350–o3351. DOI:10.1107/S1600536810048932
Mojr V., Herzig V., Budesinsky M., Cibulka R., Kraus T.: Flavin-cyclodextrin conjugates as catalysts of enantioselective sulfoxidations with hydrogen peroxide in aqueous media. Chem. Comm., 2010, 46, 7599–7601. DOI:10.1039/C0CC02562C
Kovaříček P., Eigner V., Holakovský R.: Ribbon and Pleated Sheet Formation by Anion-Directed Hydrogen Bonding In 1,4-Bis(4,5-dihydro-1H-imidazol-2-yl)benzene Salts.Structural Chemistry Communications, 2010, Vol. 1, No. 1, 8-11. http://www.factumpress.com/index.php/scc/article/viewFile/19/pdf
Jurok R., Cibulka R., Dvořáková H., Hampl F., Hodačová J.: Planar Chiral Flavinium Salts - Prospective Catalysts for Enantioselective Sulfoxidation Reactions. Eur. J. Org. Chem. 2010, 5217-5224. DOI:10.1002/ejoc.201000592
Cibulka R.: Flaviny – perspektivní katalyzátory oxidací a redukcí. Chem. Listy 2010,104, 326-333. http://chemicke-listy.cz/docs/full/2010_05_326-333.pdf
J. Budka, V. Eigner, R. Holakovský, P. Kovarícek and T. Louzilová: 25,26,27,28-Tetrapropoxycalix[4]arene-5,17-dicarbonitrile. Acta Cryst. 2010, E66, o419-o420. http://journals.iucr.org/e/issues/2010/02/00/om2307/om2307.pdf
Jiří Žurek, Radek Cibulka, Hana Dvořáková, Jiří Svoboda: N1,N10-Ethylene-bridged flavinium salts derived from L-valinol: synthesis and catalytic activity in H2O2oxidations, Tetrahedron Lett. 2010, 51, 1083-1086. DOI:10.1016/j.tetlet.2009.12.096
2009
Ménová P., Eigner V., Cibulka R., Čejka J., Dvořáková H.: 5-Ethyl-4a-methoxy-1,3-dimethyl-4a,5-dihydrobenzo[g]pteridine-2,4(1H,3H)dione. Acta Cryst. 2009, E65, o1536–o1537. DOI:10.1107/S1600536809020856
Cibulka R., Baxová L., Dvořáková H., Hampl F., Ménová P., Mojr V., Plancq B., Sayin S.: Catalytic effect of alloxazinium and isoalloxazinium salts on oxidation of sulfides with hydrogen peroxide in micellar media. Collect. Czech. Chem. Commun. 2009, 74, 973-993. DOI:10.1135/cccc2009030
2008
Jurok R., Svobodová E., Cibulka R.,Hampl F.: Reactivity in micelles - Are we really able to design micellar catalysts? Collect. Czech. Chem. Commun. 2008, 73, 127-146. DOI:10.1135/cccc20080127
2007
Baxová L., Cibulka R., Hampl F.: Organocatalytic sulfoxidation in micellar systems containing amphiphilic flavinium salts using hydrogen peroxide as terminal oxidant. J. Mol. Catal. A. 2007, 277, 53–60. DOI:10.1016/j.molcata.2007.07.027
Cibulka R., Svobodová E., König B., Ludvík J., Hampl F., Liška F.: Studium využití některých N-donorových ligandů a jejich komplexů s ionty přechodných kovů. Chem. Listy 2007, 101, 886-894. http://chemicke-listy.cz/docs/full/2007_11_886-894.pdf
2006
Celik H., Ekmekci G., Ludvík J., Pícha J., Zuman P: Electroreduction of aromatic oximes: Diprotonation, adsorption, imine formation, and substituent effects. J. Phys. Chem. B 2006, 110, 6785-6796. DOI:10.1021/jp056808t
Kivala M., Cibulka R., Hampl F.: Cleavage of 4-nitrophenyl diphenyl phosphate by isomeric quaternary pyridinium ketoximes - How can structure and lipophilicity of functional surfactants influence their reactivity in micelles and microemulsions?Collect. Czech. Chem. Commun. 2006, 71, 1642-1658. DOI:10.1135/cccc20061642
2005
Svobodová E., Cibulka R., Hampl F., Šmidrkal J., Liška F.: Metal ion transport through bulk liquid membrane mediated by cationic ligand surfactants. Collect. Czech. Chem. Commun. 2005, 70, 441-465. DOI:10.1135/cccc20050441
Chvapil M., Kielar F., Liška F., Šilhánková A., Bregel K.: Synthesis and evaluation of long-acting d-penicillamine derivatives. Connective Tissue Research 2005, 46, 242-250. DOI:10.1080/03008200500416690
Pícha J., Kuča K., Kivala M., Kohout M., Cabal J., Liška F.: A new group of monoquaternary reactivators of acetylcholinesterase inhibited by nerve agents. J. Enzyme Inhib. Med. Chem. 2005, 20, 233-237. DOI:10.1080/14756360400021858
2004
Pícha J., Cibulka R., Hampl F., Liška F., Pařík P., Pytela O.: Reactivity of p-substituted benzaldoximes in the cleavage of p-nitrophenyl acetate: kinetic and mechanism.Collect. Czech. Chem. Commun. 2004, 69, 397-413. DOI:10.1135/cccc20040397
Pícha J., Cibulka R., Liška F., Pařík P., Pytela O.: Reparametrization and/or determination of Hammett, inductive, mesomeric and AISE substituent constants for five substituents: N+(CH3)3, CH2N+(CH3)3, CH2Py, CH2SO2CH3 and PO(OCH3)2.Collect. Czech. Chem. Commun. 2004, 69, 2239-2252. DOI:10.1135/cccc20042239
Wiest O., Harrison C. B., Saettel N. J., Cibulka R., Sax M., König B.: Design, synthesis and evaluation of a biomimetic artificial photolyase model. J. Org. Chem. 2004, 69, 8183–8185. DOI:10.1021/jo0494329
Cibulka R., Vasold R. König B.: Catalytic Photooxidation of 4-Methoxybenzyl alcohol with Flavin Zinc(II) cyclen complex. Chem. Eur. J. 2004, 10, 6223–6231. DOI:10.1002/chem.200400232
2002
Ludvik J., Cibulka R.: Electroreduction of methyl azinyl ketoximes and their Ni(II), Cu(II) and Zn(II) complexes on mercury - correlation with their hydrolytic activity. Proceedings of the Fifth International Manuel M. Baizer Symposium in Honor of Professor Jean Michel Saveant, The 201th Meeting of the Electrochemical Society2002, Vol. 10, p. 142–146.
2001
Cibulka R., Císařová I., Ondráček J., Liška F., Ludvík J.: Electrochemical Reductions of Ni2+, Cu2+ and Zn2+ Complexes of Azinyl Methyl Ketoximes on Mercury. Collect. Czech. Chem. Commun. 2001, 66, 170. DOI:10.1135/cccc20010170
Kotoučová H., Cibulka R., Hampl F., Liška F.: Amphiphilic quaternary pyridinium ketoximes as functional hydrolytic micellar catalysts – does the nucleophilic function position influence their reactivity? J. Mol. Catal., 2001, 174, 59. DOI:10.1016/S1381-1169(01)00178-9
Mlíčková K., Šebela M., Cibulka R., Frébort I., Peč P., Liška F., Tanizawa K.: Inhibition of copper amine oxidases by pyridine-derived aldoximes and ketoximes. Biochimie 2001, 83(11-12), 995–1002. DOI:10.1016/S0300-9084(01)01345-1
Cibulka R., Hampl F., Kotoučová H., Páv O., Šilhánková A., Liška F.: Quaternary ketoximes – New perspective compounds for hydrolysis of toxic organophosphates.Vojenské zdrav. Listy – Suplementum- 2001, 70(1), 38–40.
Cibulka R.: Hydrolytické katalyzátory založené na komplexech alkyl(pyridin-2-yl)ketoximů. Chem. Listy 2001, 95, 809–810. http://chemicke-listy.cz/docs/full/archiv/2001/12-PDF/809-810.pdf
2000
Cibulka R., Hampl F., Kotoučová H., Mazáč J., Liška F.: Quaternary pyridinium ketoximes – New efficient micellar hydrolytic catalysts. Collect. Czech. Chem. Commun. 2000, 65, 227. DOI:10.1135/cccc20000227
Cibulka R., Liška F., Ludvík J.: Electrochemical reductions of methyl azinyl ketoximes on mercury. Collect. Czech. Chem. Commun. 2000, 65, 1630. DOI:10.1135/cccc20001630
1999
Cibulka R., Hampl F., Šmidrkal J. and Liška F.: Lipophilic N-[2-hydroxyimino-2-(pyridin-2-yl)ethyl]tialkylammonium salts – New ligands for metal ion extractions into organic solvents, Tetrahedron Lett. 1999, 40, 6849. DOI:10.1016/S0040-4039(99)01382-9
Cibulka R., Hampl F., Martinu T., Mazac J., Totevova S., Liska F.: Metal ion chelates of lipophilic alkyl diazinyl ketoximes as hydrolytic catalysts Collect. Czech. Chem. Commun. 1999, 64, 1159. DOI:10.1135/cccc19991159
1998
Cibulka R., Hampl F., Liška F.: Příprava a využití amfifilních oximů odvozených od azinů, Chem. listy 1998, 92(6), 469. http://chemicke-listy.cz/docs/full/1998_06_469-474.pdf
Kotoučová H., Mazáč J., Cibulka R., Hampl F. and Liška F: Unusual course of the p-nitrophenyl phosphate esters cleavage by 3-hydroxyiminoalkylpyridinium salts in micellar solutions, Chem. Lett. 1998 (7), 649. DOI:10.1246/cl.1998.649
1997
Cibulka R., Dvořák D., Hampl F., Liška F.: Metallomicellar hydrolytic catalysts containing ligand surfactants derived from alkyl-2-pyridinylketoxime, Collect. Czech. Chem. Commun. 1997, 62, 1342. DOI:10.1135/cccc19971342
[urlnadstranka] => [ogobrazek] => [pozadi] => [poduzel] => stdClass Object ( [38649] => stdClass Object ( [nazev] => Cibulka's Group [barva_pozadi] => cervena [uslideru] => false [text] => [iduzel] => 38649 [canonical_url] => [skupina_www] => Array ( ) [url] => [sablona] => stdClass Object ( [class] => infobox [html] => [css] => [js] => [autonomni] => 0 ) ) [39232] => stdClass Object ( [obsah] => [iduzel] => 39232 [canonical_url] => [skupina_www] => Array ( ) [url] => [sablona] => stdClass Object ( [class] => slider [html] => [css] => [js] => [autonomni] => 0 ) ) ) [iduzel] => 39235 [canonical_url] => [skupina_www] => Array ( ) [url] => /research-groups/cibulka-en/publications [sablona] => stdClass Object ( [class] => stranka [html] => [css] => [js] => [autonomni] => 1 ) ) ) [sablona] => stdClass Object ( [class] => stranka [html] => [css] => [js] => [autonomni] => 1 ) [api_suffix] => )