stdClass Object
(
[nazev] => Department of Organic Chemistry
[adresa_url] =>
[api_hash] =>
[seo_desc] =>
[jazyk] =>
[jednojazycny] =>
[barva] =>
[indexace] => 1
[obrazek] =>
[ga_force] =>
[cookie_force] =>
[secureredirect] =>
[google_verification] => UOa3DCAUaJJ2C3MuUhI9eR1T9ZNzenZfHPQN4wupOE8
[ga_account] => UA-10822215-3
[ga_domain] =>
[ga4_account] => G-VKDBFLKL51
[gtm_id] =>
[gt_code] =>
[kontrola_pred] =>
[omezeni] => 0
[pozadi1] =>
[pozadi2] =>
[pozadi3] =>
[pozadi4] =>
[pozadi5] =>
[robots] =>
[htmlheaders] =>
[newurl_domain] => 'uoch.vscht.cz'
[newurl_jazyk] => 'en'
[newurl_akce] => '[en]'
[newurl_iduzel] =>
[newurl_path] => 8548/6214/6521
[newurl_path_link] => Odkaz na newurlCMS
[iduzel] => 6521
[platne_od] => 31.10.2023 17:15:00
[zmeneno_cas] => 31.10.2023 17:15:57.546654
[zmeneno_uzivatel_jmeno] => Jan Kříž
[canonical_url] =>
[idvazba] => 7334
[cms_time] => 1713880038
[skupina_www] => Array
(
)
[slovnik] => stdClass Object
(
[logo_href] => /
[logo] =>  [google_search] => 001523547858480163194:u-cbn29rzve
[social_fb_odkaz] => https://www.facebook.com/uoch.vscht
[social_tw_odkaz] => https://twitter.com/uoch_vscht
[social_yt_odkaz] =>
[paticka_budova_a_nadpis] => BUILDING A
[paticka_budova_a_popis] => Rector,
Department of Communications,
Department of Education,
FCT Dean’s Office,
Centre for Information Services
[paticka_budova_b_nadpis] => BUILDING B
[paticka_budova_b_popis] => Department of R&D, Dean’s Offices:
FET,
FFBT,
FCE,
Computer Centre,
Department of International Relations,
Bursar
[paticka_budova_c_nadpis] => BUILDING C
[paticka_budova_c_popis] =>
Crèche Zkumavka,
General Practitioner,
Department of Economics and Management,
Department of Mathematics
[paticka_budova_1_nadpis] => NATIONAL LIBRARY OF TECHNOLOGY
[paticka_budova_1_popis] =>
[paticka_budova_2_nadpis] => CAFÉ CARBON
[paticka_budova_2_popis] =>
[paticka_adresa] => UCT Prague
[google_search] => 001523547858480163194:u-cbn29rzve
[social_fb_odkaz] => https://www.facebook.com/uoch.vscht
[social_tw_odkaz] => https://twitter.com/uoch_vscht
[social_yt_odkaz] =>
[paticka_budova_a_nadpis] => BUILDING A
[paticka_budova_a_popis] => Rector,
Department of Communications,
Department of Education,
FCT Dean’s Office,
Centre for Information Services
[paticka_budova_b_nadpis] => BUILDING B
[paticka_budova_b_popis] => Department of R&D, Dean’s Offices:
FET,
FFBT,
FCE,
Computer Centre,
Department of International Relations,
Bursar
[paticka_budova_c_nadpis] => BUILDING C
[paticka_budova_c_popis] =>
Crèche Zkumavka,
General Practitioner,
Department of Economics and Management,
Department of Mathematics
[paticka_budova_1_nadpis] => NATIONAL LIBRARY OF TECHNOLOGY
[paticka_budova_1_popis] =>
[paticka_budova_2_nadpis] => CAFÉ CARBON
[paticka_budova_2_popis] =>
[paticka_adresa] => UCT Prague
Technická 5
166 28 Prague 6 – Dejvice
IČO: 60461373 / VAT: CZ60461373
Czech Post certified digital mail code: sp4j9ch
Copyright: UCT Prague 2015
Information provided by the Department of Organic Chemistry. Technical support by the Computing Centre.
[paticka_odkaz_mail] => mailto:kundrato@vscht.cz
[social_fb_title] => Facebook of Department
[social_tw_title] => Twitter of Department
[social_yt_title] =>
[aktualizovano] => Updated
[autor] => Author
[paticka_mapa_alt] =>
[drobecky] => You are here: VŠCHT Praha – FCHT – ÚOCH
[intranet_odkaz] => http://intranet.vscht.cz/
[intranet_text] => Intranet
[logo_mobile_href] => /
[logo_mobile] =>  [mobile_over_nadpis_menu] => Menu
[mobile_over_nadpis_search] => Search
[mobile_over_nadpis_jazyky] => Languages
[mobile_over_nadpis_login] => Login
[menu_home] => Homepage
[zobraz_desktop_verzi] => switch to desktop version
[zobraz_mobilni_verzi] => switch to mobile version
[more_info] => more information
[paticka_mapa_odkaz] =>
[nepodporovany_prohlizec] => For full access, please use different browser.
[preloader] => Wait a second...
[social_in_odkaz] =>
[den_kratky_4] =>
[archiv_novinek] =>
[novinky_servis_archiv_rok] =>
[novinky_kategorie_1] =>
[novinky_kategorie_2] =>
[novinky_kategorie_3] =>
[novinky_kategorie_4] =>
[novinky_kategorie_5] =>
[novinky_archiv_url] =>
[novinky_servis_nadpis] =>
[novinky_dalsi] =>
[hledani_nadpis] => hledání
[hledani_nenalezeno] => Nenalezeno...
[hledani_vyhledat_google] => vyhledat pomocí Google
[social_li_odkaz] =>
)
[poduzel] => stdClass Object
(
[6525] => stdClass Object
(
[obsah] =>
[poduzel] => stdClass Object
(
[6532] => stdClass Object
(
[obsah] =>
[iduzel] => 6532
[canonical_url] => //uoch.vscht.cz
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
[6530] => stdClass Object
(
[obsah] =>
[iduzel] => 6530
[canonical_url] => //uoch.vscht.cz
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
[6531] => stdClass Object
(
[obsah] =>
[iduzel] => 6531
[canonical_url] => //uoch.vscht.cz
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
)
[iduzel] => 6525
[canonical_url] =>
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
[6526] => stdClass Object
(
[obsah] =>
[poduzel] => stdClass Object
(
[21923] => stdClass Object
(
[nazev] => Department of Organic Chemistry
[seo_title] => Department of Organic Chemistry
[seo_desc] =>
[autor] =>
[autor_email] =>
[obsah] =>
[mobile_over_nadpis_menu] => Menu
[mobile_over_nadpis_search] => Search
[mobile_over_nadpis_jazyky] => Languages
[mobile_over_nadpis_login] => Login
[menu_home] => Homepage
[zobraz_desktop_verzi] => switch to desktop version
[zobraz_mobilni_verzi] => switch to mobile version
[more_info] => more information
[paticka_mapa_odkaz] =>
[nepodporovany_prohlizec] => For full access, please use different browser.
[preloader] => Wait a second...
[social_in_odkaz] =>
[den_kratky_4] =>
[archiv_novinek] =>
[novinky_servis_archiv_rok] =>
[novinky_kategorie_1] =>
[novinky_kategorie_2] =>
[novinky_kategorie_3] =>
[novinky_kategorie_4] =>
[novinky_kategorie_5] =>
[novinky_archiv_url] =>
[novinky_servis_nadpis] =>
[novinky_dalsi] =>
[hledani_nadpis] => hledání
[hledani_nenalezeno] => Nenalezeno...
[hledani_vyhledat_google] => vyhledat pomocí Google
[social_li_odkaz] =>
)
[poduzel] => stdClass Object
(
[6525] => stdClass Object
(
[obsah] =>
[poduzel] => stdClass Object
(
[6532] => stdClass Object
(
[obsah] =>
[iduzel] => 6532
[canonical_url] => //uoch.vscht.cz
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
[6530] => stdClass Object
(
[obsah] =>
[iduzel] => 6530
[canonical_url] => //uoch.vscht.cz
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
[6531] => stdClass Object
(
[obsah] =>
[iduzel] => 6531
[canonical_url] => //uoch.vscht.cz
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
)
[iduzel] => 6525
[canonical_url] =>
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
[6526] => stdClass Object
(
[obsah] =>
[poduzel] => stdClass Object
(
[21923] => stdClass Object
(
[nazev] => Department of Organic Chemistry
[seo_title] => Department of Organic Chemistry
[seo_desc] =>
[autor] =>
[autor_email] =>
[obsah] => New PhD topics of our department: here
All publications of our authors (since 2015): here
[urlnadstranka] =>
[ogobrazek] =>
[pozadi] =>
[iduzel] => 21923
[canonical_url] =>
[skupina_www] => Array
(
)
[url] => /home
[sablona] => stdClass Object
(
[class] => boxy
[html] =>
[css] =>
[js] => $(function() {
setInterval(function () { $('*[data-countdown]').each(function() { CountDownIt('#'+$(this).attr("id")); }); },1000);
setInterval(function () { $('.homebox_slider:not(.stop)').each(function () { slide($(this),true); }); },5000);
});
function CountDownIt(selector) {
var el=$(selector);foo = new Date;
var unixtime = el.attr('data-countdown')*1-parseInt(foo.getTime() / 1000); if(unixtime<0) unixtime=0;
var dnu = 1*parseInt(unixtime / (3600*24)); unixtime=unixtime-(dnu*(3600*24));
var hodin = 1*parseInt(unixtime / (3600)); unixtime=unixtime-(hodin*(3600));
var minut = 1*parseInt(unixtime / (60)); unixtime=unixtime-(minut*(60));
if(unixtime<10) {unixtime='0'+unixtime;}
if(dnu<10) {unixtime='0'+dnu;}
if(hodin<10) {unixtime='0'+hodin;}
if(minut<10) {unixtime='0'+minut;}
el.html(dnu+':'+hodin+':'+minut+':'+unixtime);
}
function slide(el,vlevo) {
if(el.length<1) return false; var leva=el.find('.content').position().left; var sirka=el.width(); var pocet=el.find('.content .homebox').length-1;
var cislo=leva/sirka*-1; if(vlevo) { if(cislo+1>pocet) cislo=0; else cislo++; } else { if(cislo==0) cislo=pocet-1; else cislo--; }
el.find('.content').animate({'left':-1*cislo*sirka});
el.find('.slider_puntiky a').removeClass('selected');
el.find('.slider_puntiky a.puntik'+cislo).addClass('selected');
return false;
}
function slideTo(el,cislo) {
if(el.length<1) return false; var sirka=el.width(); var pocet=el.find('.content .homebox').length-1; if(cislo<0 || cislo>pocet) return false;
el.find('.content').animate({'left':-1*cislo*sirka});
el.find('.slider_puntiky a').removeClass('selected');
el.find('.slider_puntiky a.puntik'+cislo).addClass('selected');
return false;
}
[autonomni] => 1
)
)
[16022] => stdClass Object
(
[nazev] => Department of Organic Chemistry - Staff
[seo_title] => Department of Organic Chemistry
[seo_desc] =>
[autor] =>
[autor_email] =>
[perex] =>
[ikona] => telefon-zvoni
[obrazek] =>
[ogobrazek] =>
[pozadi] =>
[obsah] =>
| k Phonebook of the Department | |||
Professors: |
|||
|
Prof. Radek Cibulka e +420 22044 4182 d A 278c |
Head of Department | www | ResearcherID |
|
Prof. Pavel Lhoták e +420 22044 5055 d A 249 |
www | ||
|
Prof. Jaroslav Kvíčala e +420 22044 4240 d A 278d |
www | ||
|
Prof. Jiří Svoboda e +420 22044 3688 d A 278e |
www | ||
Associate Professors: |
|||
|
Assoc. Prof. Jan Budka e +420 22044 4284 d A 251 |
DOC web editor | www | |
|
Assoc. Prof. Jana Hodačová e +420 22044 4173 d A 270 |
www | ||
|
Assoc. Prof. Michal Kohout e +420 22044 5012 d A 258 |
www | ||
|
Assoc. Prof. Igor Linhart e +420 22044 4165 d A 266 |
www | ||
|
Assoc. Prof. Tomáš Tobrman e +420 22044 4245 d A 271 |
RIT | www | |
Assistant Professors: |
|||
|
Dr. Michal Himl e +420 22044 4165 d A 266 |
www | ||
|
Dr. Roman Holakovský e +420 22044 4279 d A 255 |
www | ||
|
Dr. Petr Kovaříček e +420 22044 2040 d B3 207 |
www | ||
|
Dr. Václav Kozmík e +420 22044 4118 d A 308 |
www | ||
|
Dr. Martin Krupička e +420 220 444 173 |
www | ||
|
Dr. Ondřej Kundrát e +420 22044 4280 d A 253 |
www | ResearcherID | |
|
Dr. Petra Ménová e +420 22044 3686 d A 268 |
www | ||
|
Dr. Pavla Perlíková e +420 22044 2039 d B3 206 |
www | ||
|
Dr. Markéta Rybáčková e +420 22044 4242 d A 259 |
Secretary of Department |
www | |
|
Dr. Eva Svobodová e +420 22044 4249 d A 260 |
www | ||
Technicians: |
|||
|
Květa Bártová e +420 22044 3686 d A 268 |
|||
|
Ivana Bocková, MSc. e +420 22044 4280 d A 250 |
|||
| Michaela Kadlecová
e +420 22044 4276 d A 262 b Michaela.Kadlecova@vscht.cz |
|||
|
Martina Kovandová e +420 22044 4279 d A 255 |
|||
|
Vladimír Kuneš e +420 22044 4277 d A L06 |
|||
|
Jana Netušilová e +420 22044 4164 d A 278a |
Administrative Support of Department | ||
|
Markéta Slabochová, MSc. e +420 22044 4059 d A 308 |
Economist of Department | ||
|
Helena Štenglová e +420 22044 4278 d A 257 |
|||
|
Ing. David Tetour e +420 22044 4173 d A270 |
|||
|
Marta Tokárová e +420 220 444 245 d A 271 |
|||
Essential Information for Current Students
Bachelor Students
Master Students
PhD Students
Lectures delivered by members of the Department
- Toxicology and ecology
- Organic chemistry I, II and III
- Structural analysis
- Reactivity of organic compounds
- Molecular design
- Organic synthesis I and II
- Quantum organic chemistry
- Farmacochemistry
- Organic stereochemistry
- Organometallic chemistry
- Organic chemistry of selected elements
DATA
stdClass Object
(
[nazev] =>
[seo_title] => Tobrman's Group
[seo_desc] =>
[autor] =>
[autor_email] =>
[obsah] => News
|
January 2024 Our contribution to the Science of Synthesis has been published: T. Tobrman, 9.14.5 Phospholes (Update 2024), Science of Synthesis, 2024, DOI: https://science-of-synthesis.thieme.com/app/text/?id=SD-109-00525&position=1
|
|
October 2023 Sergej Mrkobrada defended the diploma thesis.
|
|
June 2023 Denisa Skurková defended the bachelor's thesis. One-day river trip from Týnec nad Sázavou to Pikovice.
|
|
February 2023 Zdeněk Hartman was admitted to the University of Cambridge. Congratulations!
|
|
December 2022 In collaboration with Prof. Cibulka’s group, we published the preparation of arylated flavins: Marek Čubiňák, Naisargi Varma, Petr Oeser, Adam Pokluda, Tetiana Pavlovska, Radek Cibulka,
|
|
Autumn 2022 Andrea Blahovcová, Hana Hovorková and Marek Filippi joined our group.
|
|
August 2022 Aleš Krčil defended the diploma thesis.
|
|
June 2022 Soňa Poláčková a Scarlet Stefanie Vraník defended the diploma thesis.
|
|
June2022 One-day river trip from Vyššího Brod to Český Krumlov. |
|
June 2022 Karolína Šilhánová defended the bachelor's thesis. |
|
October 2019 Veronika Budková a Nikola Studničková joined our group. |
|
October 2019 We have published stereoselective synthesis of trisubstituted alkenes: J. Organomet. Chem., Stereoselective synthesis of trisubstituted alkenyl Fischer aminocarbenes through self-mediated α-haloketone olefination, https://doi.org/10.1016/j.jorganchem.2019.120971
|
|
September 2019 We have published a review on the use of indolylboronic acids in organic synthesis: Molecules, Indolylboronic Acids: Preparation and Applications. 2019, 24, 3523. https://www.mdpi.com/1420-3049/24/19/3523 |
|
July 2019 Tereza Edlová created a laboratory logo:
|
|
June 2019 |
|
January 2019: |
Current group members
Students: |
PhD students: |
Assoc. Prof.: |
|
|
Tomáš TobrmanDepartment of Organic Chemistry UCT Prague Technická 5 166 28 Praha 6 tel: +420 220 444 245 fax: +420 220 444 288 Technician: Marta Tokárová |
Our alumni:
| PhD students: | Master students: | Bachelor students: |
Visiting students: |
|
|
|
|
Artem Petrenko, MSc.
Department of Organic Chemistry
UCT Prague
Technická 5
166 28 Praha 6
tel: +420 220 444 245
fax: +420 220 444 288
petrenka@vscht.cz
PhD thesis:
C-H activation for the synthesis of functionalized pyrenes
Publications:
Oeser, P., Koudelka, J., Petrenko, A. and Tobrman, T.:
Recent Progress Concerning the N-Arylation of Indoles.
Molecules 2021, 26, 5079. DOI: 10.3390/molecules26165079
Jakub Koudelka, MSc.
Department of Organic Chemistry
UCT Prague
Technická 5
166 28 Praha 6
tel: +420 220 444 245
fax: +420 220 444 288
jakub.koudelka@vscht.cz
PhD thesis:
Cross-coupling reactions of activated C-O bonds
Publications:
Oeser, P., Koudelka, J., Petrenko, A. and Tobrman, T.:
Recent Progress Concerning the N-Arylation of Indoles.
Molecules 2021, 26, 5079. DOI: 10.3390/molecules26165079
Koudelka, J.; Tobrman, T.:
Synthesis of 2-Substituted Cyclobutanones by a Suzuki Reaction and Dephosphorylation Sequence.
Eur. J. Org. Chem. 2021, 3260-3269. DOI:10.1002/ejoc.202100464
Oeser, P.; Koudelka, J.; Dvořáková, H.; Tobrman, T.:
Formation of trisubstituted buta-1,3-dienes and α,β-unsaturated ketones via the reaction of functionalized vinyl phosphates and vinyl phosphordiamidates with organometallic reagents.
RSC Adv. 2020, 10, 35109-35120. DOI: 10.1039/D0RA07472A
Petr Oeser, MSc.
Department of Organic Chemistry
UCT Prague
Technická 5
166 28 Praha 6
tel: +420 220 444 245
fax: +420 220 444 288
petr.oeser@vscht.cz
PhD thesis:
Cross-coupling reactions of phospholes
Publications:
Oeser, P., Koudelka, J., Petrenko, A. and Tobrman, T.:
Recent Progress Concerning the N-Arylation of Indoles.
Molecules 2021, 26, 5079. DOI: 10.3390/molecules26165079
Oeser, P., Edlová, T., Čubiňák, M. and Tobrman, T.:
Transition-Metal-Free Ring-Opening Reaction of 2-Halocyclobutanols through Ring Contraction.
Eur. J. Org. Chem. 2021, 4958-4967. DOI: 10.1002/ejoc.202100837
Oeser, P.; Koudelka, J.; Dvořáková, H.; Tobrman, T.:
Formation of trisubstituted buta-1,3-dienes and α,β-unsaturated ketones via the reaction of functionalized vinyl phosphates and vinyl phosphordiamidates with organometallic reagents.
RSC Adv. 2020, 10, 35109-35120. DOI: 10.1039/D0RA07472A
 Dr. Peter Polák
Dr. Peter Polák
Department of Organic Chemistry
UCT Prague
Technická 5
166 28 Praha 6
tel: +420 220 444 245
fax: +420 220 444 288
peter.polak@vscht.cz
PhD thesis:
Bromoenol-phosphates as building blocks for regioselective synthesis of functionalized alkenes
Publications:
Polák, P.; Čejka, J.; Tobrman, T.:
Formal Transition-Metal-Catalyzed Phosphole C–H Activation for the Synthesis of Pentasubstituted Phospholes.
Org. Lett. 2020, 22, 2187─2190. DOI: 10.1021/acs.orglett.0c00359
Tobrman, T; Krupička, M.; Polák, P.; Dvořáková, H.; Čubiňák, M.; Babor, M.; Dvořák, D.:
Diastereoselective Cyclopropanation through Michael Addition‐Initiated Ring Closure between α,α‐Dibromoketones and α,β‐Unsaturated Fischer Carbene Complexes.
Eur. J. Org. Chem. 2020, 429─436. DOI: 10.1002/ejoc.201901503
Čubiňák, M.; Edlová, T.; Polák, P.; Tobrman, T.: Indolylboronic Acids: Preparation and Applications.
Molecules 2019, 24, 3523. DOI: 10.3390/molecules24193523
Tobrman, T.; Polák, P.; Čubiňák, M.; Dvořáková, H.; Dvořák, D.: Dichotomy within 1,4-addition of organolithium and Grignard reagents to α,β-unsaturated Fischer alkoxycarbenes: A new synthesis of Fischer carbenes.
Tetrahedron 2019, 75, 2175-2181. DOI: 10.1016/j.tet.2019.02.038
Polák, P.; Tobrman, T.: Novel Selective Approach to Terminally Substituted [n]Dendralenes
Eur. J. Org. Chem. 2019, 957-968. DOI:10.1002/ejoc.201801522
Polák, P.; Dvořák, D.; Tobrman, T.:
Cyanogen: A Versatile Reagent for Diversity-Oriented Synthesis
Synthesis 2017, 49, 1757-1766. DOI:10.1055/s-0036-1588410
Polák, P.; Tobrman, T.:
The synthesis of polysubstituted indoles from 3-bromo-2-indolyl phosphates
Org. Biomol. Chem. 2017, 15, 6233-6241. DOI:10.1039/C7OB01127J
Polák, P.; Tobrman, T.:
Dearomatization Strategy for the Synthesis of Arylated 2H-Pyrroles and 2,3,5-Trisubstituted 1H-Pyrroles.
Org. Lett. 2017, 19, 460-4611. DOI: 10.1021/acs.orglett.7b02219
Kotek, V.; Polák, P.; Dvořáková, H.; Tobrman, T.:
Aluminium Chloride Promoted Cross-Coupling of Trisubstituted Enol Phosphates with Organozinc Reagents En Route to the Stereoselective Synthesis of Tamoxifen and Its Analogues.
Eur. J. Org. Chem. 2016, 5037-5044. DOI:10.1002/ejoc.201600959
Polák, P.; Váňová, H.; Dvořák, D.; Tobrman, T.:
Recent Progress in Transition Metal-Catalyzed Stereoselective Synthesis of Acyclic All-Carbon Tetrasubstituted Alkenes.
Tetrahedron Lett. 2016, 57, 3684-3693 DOI:10.1016/j.tetlet.2016.07.030
Kotek, V.; Polák, P.; Tobrman, T.:
Efficient and Simple Preparation of Functionalized 1,1-Dibromoenol Phosphates
Monatshefte fuer Chemie 2016, 147, 405-412. DOI:10.1007/s00706-015-1613-6

Assoc. Prof. Tomáš Tobrman
Department of Organic Chemistry
UCT Prague
Technická 5
166 28 Praha 6
tel: +420 220 444 245
fax: +420 220 444 288
Date and place of birth
22.11. 1977, Plzeň
Work Experience
2015–today: Associate professor at the Department of Organic Chemistry, UCT Prague.
2005–2014: Assistant professor at the Department of Organic Chemistry, UCT Prague.
22.1. 2008–30.6. 2009: Post-doc position in the group of prof. E.-i. Negishi, Purdue University, In, USA, Subject: Alkyne haloboration: An Application in stereoselective synthesis of trisubstituted alkenes.
15.9. 2004 – 28.2. 2005: Research in the group of prof. Tobias Rein, KTH, Stockholm, Sweden
Subject: Asymetric Wittig reaction
Education and training
2001-2005: PhD study at the UCT Prague (Czech Republic), Subject: Modification of purine ring by utilization of reactivity of transition metals
4.-20. 7. 2001: 101st International summer course, BASF, Ludwigshafen (Germany)
1996-2001: UCT Prague (Czech Republic)
Diploma Thesis: Chelated( h2-allylamino)carbene complexes of iron, chromium and tungsten: Preparation and properties
1992-1996: High school, MSŠCH, Prague (Czech Republic)
Marek Čubiňák, MSc.
Department of Organic Chemistry
UCT Prague
Technická 5
166 28 Praha 6
tel: +420 220 444 245
fax: +420 220 444 288
marek.cubinak@vscht.cz
PhD thesis:
Stereoselective synthesis of tetrasubstituted alkenes.
Publications:
Oeser, P., Edlová, T., Čubiňák, M. and Tobrman, T.:
Transition-Metal-Free Ring-Opening Reaction of 2-Halocyclobutanols through Ring Contraction.
Eur. J. Org. Chem. 2021, 4958-4967. DOI: 10.1002/ejoc.202100837
Čubiňák, M,. Bigeon, J., Galář, P., Ondič, L. and Tobrman, T.:
The Synthesis of Tetrasubstituted Cycloalkenes Bearing π-Conjugated Substituents and Their Optical Properties.
ChemistrySelect 2021, 6, 9904-9910. DOI: 10.1002/slct.202103122
Edlová, T.; Čubiňák, M.; Tobrman, T.:
Cross-Coupling Reactions of Double or Triple Electrophilic Templates for Alkene Synthesis.
Synthesis 2021, 53, 255-266. DOI: 10.1055/s-0040-1707270
Čubiňák, M.; Tobrman, T.:
Room-Temperature Negishi Reaction of Trisubstituted Vinyl Phosphates for the Synthesis of Tetrasubstituted Alkenes.
J. Org. Chem. 2020, 85, 10728-10739. DOI: 10.1021/acs.joc.0c01254
Tobrman, T.; Krupička, M.; Polák, P.; Dvořáková, H.; Čubiňák, M.; Babor, M. ; Dvořák, D.: Diastereoselective Cyclopropanation through Michael Addition‐Initiated Ring Closure between α,α‐Dibromoketones and α,β‐Unsaturated Fischer Carbene Complexes.
Eur. J. Org. Chem. 2020, 429-436. DOI: 10.1002/ejoc.201901503
Tobrman, T.; Čubiňák, M.; Dvořák, D.: Stereoselective synthesis of trisubstituted alkenyl Fischer aminocarbenes through self-mediated α-haloketone olefination.
J. Organomet. Chem. 2019, 902, 120971. DOI 10.1016/j.jorganchem.2019.120971
Čubiňák, M.; Edlová, T.; Polák, P.; Tobrman, T.: Indolylboronic Acids: Preparation and Applications.
Molecules 2019, 24, 3523 DOI: 10.3390/molecules24193523
Tobrman, T.; Polák, P.; Čubiňák, M.; Dvořáková, H.; Dvořák, D.: Dichotomy within 1,4-addition of organolithium and Grignard reagents to α,β-unsaturated Fischer alkoxycarbenes: A new synthesis of Fischer carbenes.
Tetrahedron 2019, 75, 2175-2181. DOI: 10.1016/j.tet.2019.02.038
Čubiňák, M.; Eigner, V.; Tobrman, T.: Bench‐Stable Sulfoxide‐Based Boronates: Preparation and Application in a Tandem Suzuki Reaction.
Adv. Synth. Catal. 2018, 360, 4604-4614. DOI:10.1002/adsc.201801000
The research activities of our group are focused on the application of the reactivity of transition metals in the following areas:
Design of new reactions and processes for the preparation of heterocyclic compounds
Research activities associated with the chemistry of heterocyclic compounds can be demonstrated by the synthesis of pentasubstituted phosphols by copper-catalyzed C-H activation of 1,3,4-trisubstituted phospholes:

Polák, P.; Tobrman, T. Formal Transition-Metal-Catalyzed Phosphole C–H Activation for the Synthesis of Pentasubstituted Phospholes.
Org. Lett. 2020, 22, 2187-2190. DOI: 10.1021/acs.orglett.0c00359
Another use of transition-metal-catalyzed reactions is represented by the preparation of trisubstituted pyrroles using a dearomatization strategy:
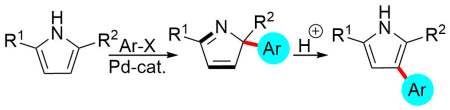
Polák, P.; Tobrman, T. Dearomatization Strategy for the Synthesis of Arylated 2H-Pyrroles and 2,3,5-Trisubstituted 1H-Pyrroles.
Org. Lett. 2017, 19, 4608-4611. DOI: 10.1021/acs.orglett.7b02219
Cross-coupling reactions of activated C-O bonds for the preparation of tetrasubstituted alkenes
The use of activated C-O bonds is an attractive alternative to organohalogen-based electrophilic templates. From a number of ways to activate C-O bonds, we pay attention to reactivity of enol-phosphates. We have recently published a new methodology for the preparation of tamoxifen that uses enol phosphate as a starting compound:
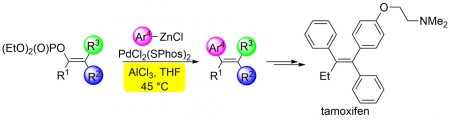
Kotek, V.; Polák, P.; Dvořáková, H.; Tobrman, T. Aluminium Chloride Promoted Cross-Coupling of Trisubstituted Enol Phosphates with Organozinc Reagents En Route to the Stereoselective Synthesis of Tamoxifen and Its Analogues
Eur. J. Org. Chem. 2016, 5037-5044. DOI: 10.1002/ejoc.201600959
Cross-coupling reactions of activated C-O bonds were also used for the preparation of 2,3-disubstituted-1,3-dienes, [3]- and [4]dendralenes:
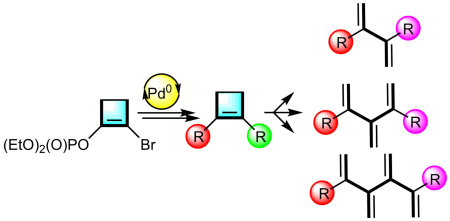
Polák, P.; Tobrman, T. Novel Selective Approach to Terminally Substituted [n]Dendralenes.
Eur. J. Org. Chem. 2019, 957-968. DOI:10.1002/ejoc.201801522
We also described the synthesis of biologically active substance starting from enol phosphate:
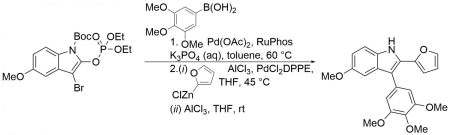
Polák, P.; Tobrman, T. The synthesis of polysubstituted indoles from 3-bromo-2-indolyl phosphates
Org. Biomol. Chem. 2017, 15, 6233-6241. DOI:10.1039/C7OB01127J
2024
2023
- J. Koudelka, M. Vosmanská and T. Tobrman, Synthesis of Sulfonic Acid Salts and Sulfonic Acids by Advanced Cross-Coupling Reaction of Vinyl Sulfonates. ChemistrySelect 2023, 8, e202304246. DOI:10.1002/slct.202304246
- T. Tobrman, Vinyl Esters and Vinyl Sulfonates as Green Alternatives to Vinyl Bromide for the Synthesis of Monosubstituted Alkenes via Transition-Metal-Catalyzed Reactions. Chemistry 2023, 5, 2288–2321. DOI:10.3390/chemistry5040153
- L. Koláčná, P. Polák, A. Liška, T. Tobrman and J. Ludvík, Pentasubstituted Phospholes with extended π-conjugated Arm – Synthesis, electrochemistry, spectra and quantum chemical calculations. Electrochim. Acta 2023, 468, 143073. DOI: 10.1016/j.electacta.2023.143073
- M. Čubiňák, N. Varma, P. Oeser, A. Pokluda, T. Pavlovska, R. Cibulka, M. Sikorski, T. Tobrman, Tuning the Photophysical Properties of Flavins by Attaching an Aryl Moiety via Direct C–C Bond Coupling. J. Org. Chem. 2023, 88, 218–229. DOI: 10.1021/acs.joc.2c02168
2022
- Tobrman, T.; Mrkobrada, S., Palladium-Catalyzed Cross-Coupling Reactions of Borylated Alkenes for the Stereoselective Synthesis of Tetrasubstituted Double Bond. Organics 2022, 3, 210.10.3390/org3030017
- Shishkanova, T. V.; Tobrman, T.; Otta, J.; Broncová, G.; Fitl, P.; Vrňata, M., Substituted polythiophene-based sensor for detection of ammonia in gaseous and aqueous environment. J. Mater. Sci. 2022, 57, 17870–17882. DOI: 10.1007/s10853-022-07694-8
- Oeser, P.; Petrenko, A.; Edlová, T.; Čubiňák, M.; Koudelka, J.; Tobrman, T., Halocyclobutanol Dehydration En Route to Halocyclobutenes. Synthesis 2022, 54, 3239–3248. DOI: 10.1055/a-1794-0685
- Koláčná, L.; Klíma, J.; Polák, P.; Tobrman, T.; Liška, A.; Ludvík, J., Electrochemical, EPR, and computational study of pyrene conjugates–precursors for novel type of organic semiconductors. J. Solid State Electrochem. 2022, 26, 503–514. DOI: 10.1007/s10008-021-05094-7
2021
- Oeser, P; Koudelka, J.; Petrenko, A.; T. Tobrman, Recent Progress Concerning the N-Arylation of Indoles. Molecules 2021, 26, 5079. DOI: 10.3390/molecules26165079
- Čubiňák, M.; Bigeon, J.; Galář, P.; Ondič, L.; Tobrman, T., The Synthesis of Tetrasubstituted Cycloalkenes Bearing π-Conjugated Substituents and Their Optical Properties. ChemistrySelect 2021, 6, 9904–9910. DOI: 10.1002/slct.202103122
- Oeser, P.; Edlová, T.; Čubiňák, M.; Tobrman, T., Transition-Metal-Free Ring-Opening Reaction of 2-Halocyclobutanols through Ring Contraction. Eur. J. Org. Chem. 2021, 4958–4967. DOI: 10.1002/ejoc.202100837
- Tobrman, T., Synthesis of 2-Substituted Cyclobutanones by a Suzuki Reaction and Dephosphorylation Sequence. Eur. J. Org. Chem. 2021, 3260–3269 DOI:10.1002/ejoc.202100464
- Tobrman, T., Substrate-Controlled Regioselective Bromination of 1,2-Disubstituted Cyclobutenes: An Application in the Synthesis of 2,3-Disubstituted Cyclobutenones.
J. Org. Chem. 2021, 86, 5820–5831 DOI:10.1021/acs.joc.1c00261 - ; Tobrman, T.; J
- Edlová, T.; Čubiňák, M.; Tobrman, T., Cross-Coupling Reactions of Double or Triple Electrophilic Templates for Alkene Synthesis. Synthesis 2021, 53, 255–266. DOI: 10.1055/s-0040-1707270
2020
- Oeser, P.; Koudelka, J.; Dvořáková, H.; Tobrman, T., Formation of trisubstituted buta-1,3-dienes and α,β-unsaturated ketones via the reaction of functionalized vinyl phosphates and vinyl phosphordiamidates with organometallic reagents. RSC Adv. 2020, 10, 35109–35120. DOI: 10.1039/D0RA07472A
- Čubiňák, M.; Tobrman, T., Room-Temperature Negishi Reaction of Trisubstituted Vinyl Phosphates for the Synthesis of Tetrasubstituted Alkenes. J. Org. Chem. 2020, 85, 10728–10739. DOI: 10.1021/acs.joc.0c01254
- Polák, P.; Čejka, J.; Tobrman, T., Formal Transition-Metal-Catalyzed Phosphole C–H Activation for the Synthesis of Pentasubstituted Phospholes. Org. Lett. 2020, 22, 2187–2190. DOI: 10.1021/acs.orglett.0c00359
- Tobrman, T; Krupička, M.; Polák, P.; Dvořáková, H.; Čubiňák, M.; Babor, M.; Dvořák, D., Diastereoselective Cyclopropanation through Michael Addition‐Initiated Ring Closure between α,α‐Dibromoketones and α,β‐Unsaturated Fischer Carbene Complexes. Eur. J. Org. Chem. 2020, 429–436. DOI: 10.1002/ejoc.201901503
- Guricová, M.; Tobrman, T; Pižl, M.; Žižková, S.; Hoskovcová, I.; Dvořák, D., Synthesis, characterisation and electrochemical properties of Cr(0) aminocarbene complexes containing condensed heteroaromatic moiety. J. Organomet. Chem. 2020, 905, 121023 DOI: 10.1016/j.jorganchem.2019.121023
2019
- Tobrman, T.; Čubiňák, M.; Dvořák, D., Stereoselective synthesis of trisubstituted alkenyl Fischer aminocarbenes through self-mediated α-haloketone olefination. J. Organomet. Chem. 2019, 902, 120971. DOI: 10.1016/j.jorganchem.2019.120971
- Čubiňák, M.; Edlová, T.; Polák, P.; Tobrman, T., Indolylboronic Acids: Preparation and Applications.
Molecules 2019, 24, 3523 DOI: 10.3390/molecules24193523 - Váňová, H.; Tobrman, T.; Babor, M.; Dvořák, D., Reaction of lithiated thiophene-derived aminocarbene complexes with inorganic halides: Preparation of a heteroatom containing mono- and multicarbene complexes. J. Organomet. Chem. 2019, 882, 90–95 DOI: 10.1016/j.jorganchem.2018.12.015
- Tobrman, T.; Polák, P.; Čubiňák, M.; Dvořáková, H.; Dvořák, D., Dichotomy within 1,4-addition of organolithium and Grignard reagents to α,β-unsaturated Fischer alkoxycarbenes: A new synthesis of Fischer carbenes. Tetrahedron 2019, 75, 2175–2181 DOI: 10.1016/j.tet.2019.02.038
- Polák, P.; Tobrman, T., Novel Selective Approach to Terminally Substituted [n]Dendralenes
Eur. J. Org. Chem. 2019, 957–968. DOI:10.1002/ejoc.201801522
2018
- Čubiňák, M.; Eigner, V.; Tobrman, T., Bench-Stable Sulfoxide-Based Boronates: Preparation and Application in a Tandem Suzuki Reaction. Adv. Synth. Catal. 2018, 360, 4604–4614 DOI: 10.1002/adsc.201801000
2017
- Polak, P.; Dvorak, D.; Tobrman, T., Cyanogen: A Versatile Reagent for Diversity-Oriented Synthesis
Synthesis 2017, 49, 1757–1766 DOI:10.1055/s-0036-1588410 - Polak, P.; Tobrman, T., The synthesis of polysubstituted indoles from 3-bromo-2-indolyl phosphates. Org. Biomol. Chem. 2017, 15, 6233–6241 DOI:10.1039/C7OB01127J
- Polak, P.; Tobrman, T., Dearomatization Strategy for the Synthesis of Arylated 2H-Pyrroles and 2,3,5-Trisubstituted 1H-Pyrroles. Org. Lett. 2017, 19, 460–4611 DOI: 10.1021/acs.orglett.7b02219
- Šimůnková, N.; Tobrman, T.; Eigner, V.; Dvořák, D., A Study on the Intramolecular Mitsunobu Reaction of N6-(ω-hydroxyalkyl)adenines. J. Heterocyclic Chem 2017, asap DOI: 10.1002/jhet.2982
2016
- Váňová, H.; Tobrman, T.; Hoskovcova, I.; Dvořák, D., Modular synthesis of Fischer biscarbene complexes of chromium. Organometallics 2016, 35, 2999–3006. DOI:10.1021/acs.organomet.6b00527
- Kotek, V.; Polák, P.; Dvořáková, H.; Tobrman, T., Aluminium Chloride Promoted Cross-Coupling of Trisubstituted Enol Phosphates with Organozinc Reagents En Route to the Stereoselective Synthesis of Tamoxifen and Its Analogues. Eur. J. Org. Chem. 2016, 5037–5044. DOI:10.1002/ejoc.201600959
- Polák, P.; Váňová, H.; Dvořák, D.; Tobrman, T., Recent Progress in Transition Metal-Catalyzed Stereoselective Synthesis of Acyclic All-Carbon Tetrasubstituted Alkenes. Tetrahedron Lett. 2016, 57, 3684–3693. DOI:10.1016/j.tetlet.2016.07.030
- Kotek, V.; Polák, P.; Tobrman, T., Efficient and Simple Preparation of Functionalized 1,1-Dibromoenol Phosphates. Monat. Chem. 2016, 147, 405–412. DOI:10.1007/s00706-015-1613-6
2015
- Kotek V., Dvořáková H., Tobrman T., Modular and highly stereoselective approach to all-carbon tetrasubstituted alkenes. Org. Lett., 2015, 17, 608–611. DOI: 10.1021/ol503624v
- Křováček M., Dvořák D.: Synthesis of potentially biologically active 6-(1,3-butadiynyl)purines. J. Heterocyclic Chem., 2015, 52, 40–47. DOI: 10.1002/jhet.1938
- Tobrman T., Jurásková I., Dvořák D., Negishi cross-coupling reaction as a simple and efficient route to functionalized amino and alkoxy carbene complexes of chromium, molybdenum, and tungsten. Organometallics, 2014, 33, 6593–6603. DOI: 10.1021/om500925m
- Kvapilová H., Eigner V., Hoskovcová I., Tobrman T., Čejka J., Záliš S., Structural flexibility of 2-hetaryl chromium aminocarbene complexes: Experimental and theoretical evidence. Inorg. Chim. Acta, 2014, 421, 439–445. DOI: 10.1016/j.ica.2014.06.028
- Vrzal L., Flídrová K., Tobrman T, Dvořáková H., Lhoták, P., Use of residual dipolar couplings in conformational analysis of meta-disubstituted calix[4]arenes. Chem. Commun., 2014, 50, 7590–7592. DOI: 10.1039/C4CC02274B
- Tobrman T, Jurásková I., Váňová H., Dvořák D, Lithiation of chromium and tungsten aminocarbene complexes: An easy approach to functionalized aminocarbene complexes. Organometallics, 2014, 33, 2990–2996. DOI: 10.1021/om500182p
- Tobrman T., Dvořák D., Regioselective and facile synthesis of 7,9-dialkyl-8-oxopurines from 7,9-dialkyl-7,8-dihydropurines: Total synthesis of Heteromines I and J. Synthesis, 2014, 46, 660–668. DOI: 10.1055/s-0033-1340499
- Maryška M., Chudíková N., Kotek V., Dvořák D., Tobrman T., Regioselective and efficient synthesis of N7-substituted adenines, guanines, and 6-mercaptopurines. Monatsh. Chem. 2013, 144, 501–507. DOI: 10.1007/s00706-012-0899-x
- Koukal P., Dvořáková H., Dvořák D., Tobrman T., Palladium-Catalyzed Claisen Rearrangement of Allyloxypurines. Chem. Pap., 2013, 67, 3–8. DOI: 10.2478/s11696-012-0239-y
- Metelková R., Tobrman T., Kvapilová H., Hoskovcová I., Ludvík J., Synthesis, characterization and electrochemical investigation of hetaryl chromium(0) aminocarbene complexes. Electrochim. Acta, 2012, 82, 470–477. DOI: 10.1016/j.electacta.2012.05.027
- Kotek V., Tobrman T., Dvořák D., Highly Efficient and Broad-Scope Protocol for the Preparation of 7-Substituted 6-halopurines via N9-Boc-Protected 7,8-dihydropurines. Synthesis 2012, 44, 610–618. DOI: 10.1055/s-0031-1290068
- Hoskovcová I., Zvěřinová R., Roháčová J., Dvořák D., Tobrman T., Záliš S., Ludvík J, Fischer aminocarbene complexes of chromium and iron: Anomalous electrochemical reduction of p-carbonyl substituted derivatives. Electrochim. Acta 2011, 56, 6853–6859. DOI: 10.1016/j.electacta.2011.05.096
- Tobrmanová M., Tobrman T., Dvořák D., Pd-Catalyzed Allylic Substitution of Purin-8-yl(allyl) Acetate: Route to (E)-Alkenylpurines. Collect. Czech. Chem. Commun. 2011, 76, 311–326. DOI: 10.1135/cccc2011030
- Negishi E.-i., Tobrman T., Rao H., Xu S., Lee Ch.-T., Highly (98 %) Selective Alkene Synthesis of Wide Applicability via Fluride-Promoted Pd-Catalyzed Cross-Coupling of Alkenylboranes. Isr. J. Chem. 2010, 50, 696–701. DOI: 10.1002/ijch.201000051
- Kotek V., Chudíková N., Tobrman T., Dvořák D., Selective Synthesis of 7-substituted Purines via 7,8-dihydropurines. Org. Lett. 2010, 12, 5724–5727. DOI: 10.1021/ol1025525
- Pařík P., Kulhánek J., Ludwig M., Wagner R., Rotrekl I., Drahoňovský D., Meca L., Šmídková M., Tobrman T., Dvořák D., Acidity of Benzoic Acids Bearing the (CO)5Cr=CN(CH3)2 Group. Organometallics 2010, 29, 4135–4138. DOI: 10.1021/om100608x
- Hoskovcová I., Roháčová J., Dvořák D., Tobrman T., Záliš S., Zvěřinová R., Ludvík J., Synthesis and electrochemical study of iron, chromium and tungsten aminocarbenes: Role of ligand structure and central metal nature. Electrochim. Acta, 2010, 55, 8341–8351. DOI: 10.1016/j.electacta.2010.02.057
- Klečka M., Křováček M., Tobrman T., Dvořák D., Synthesis of (E)-6-Alkenylpurines via Pd-Catalyzed Stannation/Protodestannation Tandem Process of Alkynylpurines. Collect. Czech. Chem. Commun. 2010, 75, 313–332. DOI: 10.1135/cccc2009563
- Wang C., Xu Z., Tobrman T., Negishi E.-i., Arylethyne Bromoboration-Negishi Coupling Route to E- or Z-Aryl-Substituted Trisubstituted Alkenes of >98% Isomeric Purity. New Horizon in the Highly Selective Synthesis of Trisubstituted Alkenes. Adv. Synth Catal., 2010, 352, 627–631. DOI: 10.1002/adsc.200900766
- Wang C., Tobrman T., Xu Z., Negishi E.-i., Highly Regio- and Stereoselective Synthesis of (Z)-Trisubstituted Alkenes via Propyne Bromoboration and Tandem Pd-Catalyzed Cross-Coupling. Org. Lett. 2009, 11, 4092–4095. DOI: 10.1021/ol901566e
- Keder R., Dvořáková H., Dvořák D., New Approach to the Synthesis of N-7-Arylguanines and N-7-Aryladenines. Eur. J. Org. Chem. 2009, 1522–1531. DOI: 10.1002/ejoc.200801002
- Tobrman T., Dvořák D., Heck reactions of 6- and 2-halopurines. Eur. J. Org. Chem. 2008, 2923–2928. DOI: 10.1002/ejoc.200800091
- Krouželka J., Linhart I., Tobrman T., Synthesis of 3-(2-hydroxy-1-phenylethyl)- and 3-(2-hydroxy-2-phenylehtyl)adenine, DNA adducts derived from styrene. J. Heterocyclic Chem. 2008, 45, 789–795. DOI: 10.1002/chin.200838155
- Tobrman T., Štepnička P., Císařová I., Dvořák D., Preparation and crystal structures of purine 2,2’-, 6,6’-, and 8,8’-dimers. Eur. J. Org. Chem. 2008, 2167–2174. DOI: 10.1002/ejoc.200800017
- Tobrman T., Dvořák D., Reductive Dimerization of 2- and 6-Iodopurines: Side reaction in Pd-Catalyzed Cross Coupling of Iodopurines. Collect. Czech Chem. Comm. 2007, 72, 1365–1374. DOI: 10.1135/cccc20071365
- Tobrman T., Dvořák D., Selective magnesiation of chloro-iodopurines: An efficient approach to new purine derivatives. Org. Lett. 2006, 8, 1291–1294. DOI: 10.1021/ol053013w
- Tobrman T., Meca L., Dvořáková H., Černý J., Dvořák D., Study of the Origin of the Hindered Rotation of an Aryl Ring in the Chromium Aminocarbene Complexes Bearing Aromatic Ring Atteched to the Carbene Carbon Atom. Organometalics 2006, 25, 5540–5548. DOI: 10.1021/om0605837
- Klečka M., Tobrman T., Dvořák D., Cu(I)-catalyzed coupling of (9-benzylpurin-6-yl)magnesium chloride with allyl halides: an approach to 6-allylpurine derivatives. Collect. Czech Chem. Comm. 2006, 71, 1221–1228. DOI: 10.1135/cccc20061221
- Hoskovcová I., Roháčová J., Meca L., et al., Electrochemistry of chromium(0)-aminocarbene complexes: The use of intramolecular interaction LFER for characterization of the oxidation and reduction centre of the complex. Electrochim. Acta 2005, 50, 4911–4915. DOI: 10.1016/j.electacta.2004.12.047
- Drahoňovský D., Štěpnička P., Dvořák D., P-chiral 2-{1 '-[butyl(phenyl)phosphanyl]ferrocen-1-yl} -4-isopropyl-4,5-dihydrooxazoles: A second chirality center in catalytic system. Collect. Czech Chem. Comm. 2005, 70, 361–369. DOI: 10.1135/cccc20050361
- Meca L., Dvořák D., Ludvík J., et al., Synthesis, structures, and electrochemistry of group 6 aminocarbenes with a P-chelating 1'-(Diphenylphosphino)ferrocenyl substituent Organometallics 2004, 23, 2541–2551. DOI: 10.1021/om040007f
- Tobrman T., Dvořák D., 'Reductive Heck reaction' of 6-halopurines. Tetrahedron Lett. 2004, 45, 273–276. DOI: 10.1016/j.tetlet.2003.10.181
- Tobrman T., Dvořák D., 6-Magnesiated purines: Preparation and reaction with aldehydes. Org. Lett. 2003, 5, 4289–4291. DOI: 10.1021/ol0355027
- Hocek M., Dvořák D., Havelková M., Covalent analogues of nucleobase-pairs. Nucleoside Nucleotides & Nucleic Acids 2003, 22, 775–777. DOI: 10.1081/NCN-120022632
- Meca L., Císařová I., Dvořák D., Thermolysis of iron N-allylaminocarbene complexes: Formation of eta(3)-1-azaallylirontricarbonyl complexes. Synthetic and theoretical study. Organometallics 2003, 22, 3703–3709. DOI: 10.1021/om0301280
- Drahoňovský D., Borgo V., Dvořák D., Fischer chromium carbene complexes as nucleophiles in palladium-catalyzed allylic substitution reactions. Tetrahedron Lett. 2002, 43, 7867–7869. DOI: 10.1016/S0040-4039(02)01913-5
- Havelková M., Dvořák D., Hocek M., Covalent analogues of DNA base-pairs and triplets. Part 3: Synthesis of 1 ,4-and 1,3-bis(purin-6-yl)benzenes and 1-(1,3-dimethyluracil-5-yl)-3 or 4-(purin-9-yl)benzenes. Tetrahedron 2002, 58, 7431–7435. DOI: 10.1016/S0040-4020(02)00833-5
- Havránek M., Dvořák D., 3,3-disubstituted allyl alcohols from palladium-catalyzed coupling of hydroaluminated propargyl alcohols with aryl iodides. J. Org. Chem. 2002, 67, 2125–2130. DOI: 10.1021/jo0162235
- Vyklický L., Dvořáková H., Dvořák D., Iron aminocarbene complexes containing a double C=C bond in the N-substituent: Preparation and reactivity. Organometallics 2001, 20, 5419–5424. DOI: 10.1021/om010533w
- Havelková M., Dvořák D., Hocek M., The Suzuki-Miyaura cross-coupling reactions of 2-, 6-or 8-halopurines with boronic acids leading to 2-, 6-or 8-aryl- and -alkenylpurine derivatives. Synthesis 2001, 1704–1710. DOI: 10.1055/s-1999-2753
- Drahoňovský D., Císařová I., Štěpnička P., et al., An alternative approach to chiral 2-[1 '-(diphenylphosphanyl)ferrocenyl]-4,5-dihydrooxazoles. Collect. Czech. Chem. Commun. 2001, 66, 588–604. DOI: 10.1135/cccc20010588
- Rotrekl I., Vyklický L., Dvořák D., Reaction of iron aminocarbene complexes with electronically deficient alkenes. J. Organometallic Chem. 2001, 617, 329–333. DOI: 10.1016/S0022-328X(00)00707-5
- Havelková M., Studenovský M., Dvořák D., N-1-substituted hypoxanthine derivatives from the reaction of 6-halopurines with Michael acceptors under the conditions of Heck reaction. Collect. Czech. Chem. Commun. 2000, 65, 797–804. DOI: 10.1135/cccc20000797
- Havránek M., Dvořák D., 3-(tributylstannyl)allyl alcohols: Useful building blocks for solid-phase synthesis of skipped dienes and trienes. Collect. Czech. Chem. Commun. 2000, 65, 434–454. DOI: 10.1135/cccc20000434
- Havelková M., Hocek M., Česnek M., et al., The Suzuki-Miyaura cross-coupling reactions of 6-halopurines with boronic acids leading to 6-aryl- and 6-alkenylpurines. Synlett 1999, 1145–1147. DOI: 10.1055/s-1999-2753
- Suchý P., Dvořák D., Havelková M., Unexpected course of the reaction of 1,3-bis(dimethylamino)trimethinium perchlorate with 3-substituted prop-2-ynals leading to 1-aryl-2,4,6-triformylbenzenes. Collect. Czech. Chem. Commun. 1999, 64, 119–129. DOI: 10.1135/cccc19990119
- Dvořáková H., Dvořák D., Holý A., Synthesis of acyclic nucleotide analogues derived from 6-(sec- or tert-alkyl)purines via coupling of 6-chloropurine derivatives with organocuprates. Collect. Czech. Chem. Commun. 1998, 63, 2065–2074. DOI: 10.1135/cccc19982065
- Havránek M., Dvořák D., Transmetallation from aluminum to tin: A facile preparation of tributylstannylprop-2-en-1-ols. Synthesis 1998, 1264–1268. DOI: 10.1055/s-1998-6101
- Dvořák D., Ludwig M., [(N,N-dimethylamino)carboxymethylene]pentacarbonyl-chromium(0): The first Fischer carbene complex with a free carboxyl group attached directly to the carbene atom. Organometallics 1998, 17, 3627–3629. DOI: 10.1021/om980082o
- Dvořák D., Transition metals in organic synthesis - Basic principles. Chemické Listy 1997, 91, 216–226.
- Arnold Z., Dvořák D., Havránek M., Convenient preparation of 1,3-bis(dimethylamino)trimethinium perchlorate, tetrafluoroborate and hexafluorophosphate. Collect. Czech. Chem. Commun. 1996, 61, 1637–1641. DOI: 10.1135/cccc19961637
- Wagner R., Dvořák D., Holý A.: New application of the Stille coupling in the synthesis of 5-substituted uracils. Collect. Czech. Chem. Commun. 1996, 61, S118–S119 Sp. Iss. SI. DOI: 10.1135/cccc1996s118
- Dvořáková H., Dvořák D., Holý A., Coupling of 6-chloropurines with organocuprates derived from Grignard reagents: A convenient route to sec and tert 6-alkylpurines. Tetrahedron Lett. 1996, 37, 1285–1288. DOI: 10.1016/0040-4039(95)02375-5