Tuning the Photophysical Properties of Flavins by Attaching an Aryl Moiety via Direct C–C Bond Coupling
Abstract: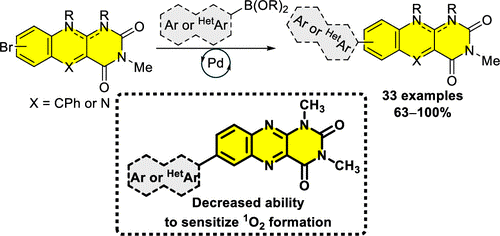 Palladium-catalyzed Suzuki reactions of brominated flavin derivatives (5-deazaflavins, alloxazines, and isoalloxazines) with boronic acids or boronic acid esters that occur readily under mild conditions were shown to be an effective tool for the synthesis of a broad range of 7/8-arylflavins. In general, the introduction of an aryl/heteroaryl group by means of a direct C–C bond has been shown to be a promising approach to tuning the photophysical properties of flavin derivatives. The aryl substituents caused a bathochromic shift in the absorption spectra of up to 52 nm and prolonged the fluorescence lifetime by up to 1 order of magnitude. Moreover, arylation of flavin derivatives decreased their ability to generate singlet oxygen.
Palladium-catalyzed Suzuki reactions of brominated flavin derivatives (5-deazaflavins, alloxazines, and isoalloxazines) with boronic acids or boronic acid esters that occur readily under mild conditions were shown to be an effective tool for the synthesis of a broad range of 7/8-arylflavins. In general, the introduction of an aryl/heteroaryl group by means of a direct C–C bond has been shown to be a promising approach to tuning the photophysical properties of flavin derivatives. The aryl substituents caused a bathochromic shift in the absorption spectra of up to 52 nm and prolonged the fluorescence lifetime by up to 1 order of magnitude. Moreover, arylation of flavin derivatives decreased their ability to generate singlet oxygen.
Highly Chemoselective Catalytic Photooxidations by Using Solvent as a Sacrificial Electron Acceptor
Abstract:
Catalyst recovery is an integral part of photoredox catalysis. It is often solved by adding another component-a sacrificial agent-whose role is to convert the catalyst back into its original oxidation state. However, an additive may cause a side reaction thus decreasing the selectivity and overall efficiency. Herein, we present a novel approach towards chemoselective photooxidation reactions based on suitable solvent-acetonitrile acting simultaneously as an electron acceptor for catalyst recovery, and on anaerobic conditions. This is allowed by the unique properties of the catalyst, 7,8-dimethoxy-3-methyl-5-phenyl-5-deazaflavinium chloride existing in both strongly oxidizing and reducing forms, whose strength is increased by excitation with visible light. Usefulness of this system is demonstrated in chemoselective dehydrogenations of 4-methoxy- and 4-chlorobenzyl alcohols to aldehydes without over-oxidation to benzoic acids achieving yields up to 70 %. 4-Substituted 1-phenylethanols were oxidized to ketones with yields 80–100 % and, moreover, with yields 31-98 % in the presence of benzylic methyl group, diphenylmethane or thioanisole which are readily oxidized in the presence of oxygen but these were untouched with our system. Mechanistic studies based on UV-Vis spectro-electrochemistry, EPR and time-resolved spectroscopy measurements showed that the process involving an electron release from an excited deazaflavin radical to acetonitrile under formation of solvated electron is crucial for the catalyst recovery.
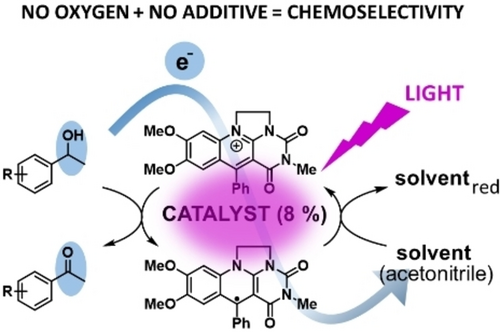 |
New way to drive photoredox catalysis: Highly chemoselective photooxidations of benzylic alcohols to carbonyl compounds in the presence of various easily-oxidizable groups are possible in a simple oxygen-free system consisting of a substrate, unique deazaflavinium catalyst and acetonitrile which acts simultaneously as a sacrificial electron acceptor and solvent. |
Tuning Deazaflavins Towards Highly Potent Reducing Photocatalysts Guided by Mechanistic Understanding – Enhancement of the Key Step by the Internal Heavy Atom Effect
Abstract:
Deazaflavins are well suited for reductive chemistry acting via a consecutive photo-induced electron transfer, in which their triplet state and semiquinone – the latter is formed from the former after electron transfer from a sacrificial electron donor – are key intermediates. Guided by mechanistic investigations aiming to increase intersystem crossing by the internal heavy atom effect and optimising the concentration conditions to avoid unproductive excited singlet reactions, we synthesised 5-aryldeazaflavins with Br or Cl substituents on different structural positions via a three-component reaction. Bromination of the deazaisoalloxazine core leads to almost 100 % triplet yield but causes photo-instability and enhances unproductive side reactions. Bromine on the 5-phenyl group in ortho position does not affect the photostability, increases the triplet yield, and allows its efficient usage in the photocatalytic dehalogenation of bromo- and chloroarenes with electron-donating methoxy and alkyl groups even under aerobic conditions. Reductive powers comparable to lithium are achieved.
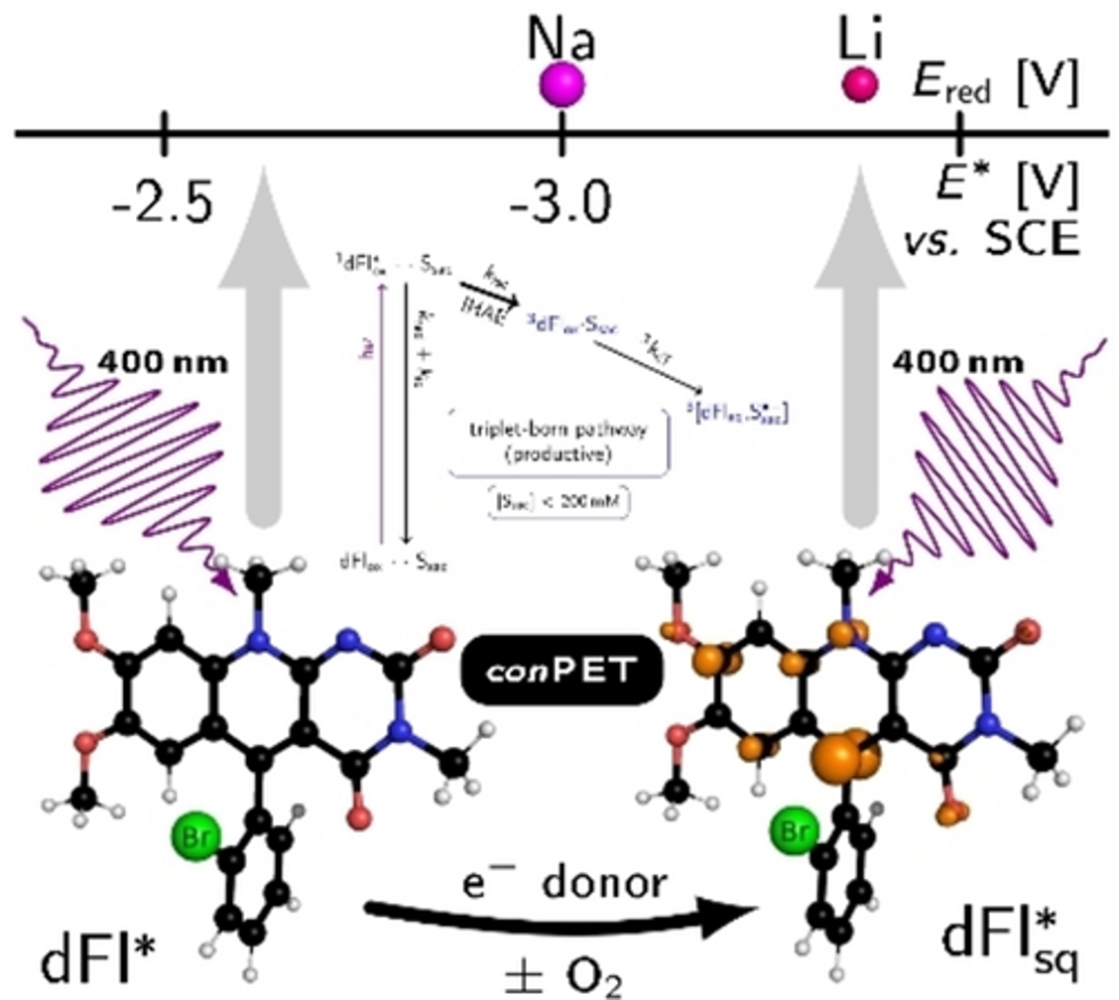 |
Inspired by nature and based on mechanistic understanding, we designed deazaflavins (dFl) with reducing powers comparable to Li which function via the consecutive photo-induced electron transfer (conPET) mechanism independently on molecular oxygen. The internal heavy atom effect (IHAE) by introducing bromine into the photocatalyst enhances considerably the key triplet pathway via a triplet-born radical pair. Furthermore, an optimal concentration of the sacrificial electron donor (Ssac) is required for bypassing the unproductive reaction via the singlet-born radical pair. |
Photophysical properties of alloxazine derivatives with extended aromaticity – Potential redox-sensitive fluorescent probe
DOI: 10.1016/j.saa.2022.120985
Abstract: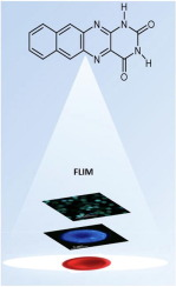 The spectral and photophysical properties of two four-ring alloxazine derivatives, naphtho[2,3-g]pteridine-2,4(1H,3H)-dione (1a) and 1,3-dimethylnaphtho[2,3-g]pteridine-2,4(1H,3H)-dione, (1b) were studied. The propensity of 1a for excited-state proton transfer reactions in the presence of acetic acid as a catalyst was also studied, showing no signature of the reaction occurring. In addition, quenching of 1a fluorescence by acetic acid was investigated. Singlet and triplet states and spectral data for 1a and 1b were calculated using density functional theory TD-DFT at B3LYP/6-31G(d) and UB3LYP levels. Finally, fluorescence lifetime imaging microscopy (FLIM) using 1a and 1b as fluorescence probes was applied to in vitro human red blood cells (RBCs) with and without tert-butyl hydroperoxide (TB) as an oxidising agent. To evaluate and compare the effects of 1a and 1b on the redox properties of RBCs, the fluorescence lifetime, amplitude and fractional intensities were calculated, and phasor plot analysis was performed. The results obtained show the appearance of a new proximal cluster in the phasor fingerprint of RBCs in the presence of 1b and a shorter fluorescence lifetime of RBCs in the presence of 1a.
The spectral and photophysical properties of two four-ring alloxazine derivatives, naphtho[2,3-g]pteridine-2,4(1H,3H)-dione (1a) and 1,3-dimethylnaphtho[2,3-g]pteridine-2,4(1H,3H)-dione, (1b) were studied. The propensity of 1a for excited-state proton transfer reactions in the presence of acetic acid as a catalyst was also studied, showing no signature of the reaction occurring. In addition, quenching of 1a fluorescence by acetic acid was investigated. Singlet and triplet states and spectral data for 1a and 1b were calculated using density functional theory TD-DFT at B3LYP/6-31G(d) and UB3LYP levels. Finally, fluorescence lifetime imaging microscopy (FLIM) using 1a and 1b as fluorescence probes was applied to in vitro human red blood cells (RBCs) with and without tert-butyl hydroperoxide (TB) as an oxidising agent. To evaluate and compare the effects of 1a and 1b on the redox properties of RBCs, the fluorescence lifetime, amplitude and fractional intensities were calculated, and phasor plot analysis was performed. The results obtained show the appearance of a new proximal cluster in the phasor fingerprint of RBCs in the presence of 1b and a shorter fluorescence lifetime of RBCs in the presence of 1a.
Amide Bond Formation via Aerobic Photooxidative Coupling of Aldehydes with Amines Catalyzed by a Riboflavin Derivative
doi.org/10.1021/acs.orglett.1c02391
 Abstract:
Abstract:
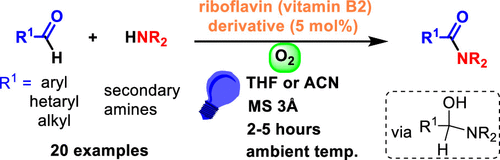
We report an effective, operationally simple, and environmentally friendly system for the synthesis of tertiary amides by the oxidative coupling of aromatic or aliphatic aldehydes with amines mediated by riboflavin tetraacetate (RFTA), an inexpensive organic photocatalyst, and visible light using oxygen as the sole oxidant. The method is based on the oxidative power of an excited flavin catalyst and the relatively low oxidation potential of the hemiaminal formed by amine to aldehyde addition.
Flavin-Helicene Amphiphilic Hybrids: Synthesis, Characterization, and Preparation of Surface-Supported Films
doi.org/10.1002/cplu.202100092
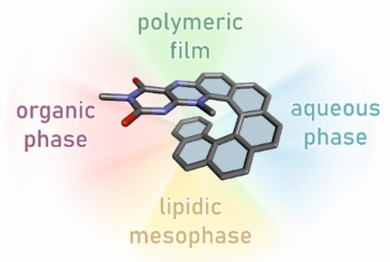 The complex characterization of a flavo[7]helicene conjugate is reported in this work. This conjugate combines inherent helical chirality with redox activity, which was studied in solution (both aqueous and organic phases), in layers (electropolymers), and in the solid state (a single crystal).
The complex characterization of a flavo[7]helicene conjugate is reported in this work. This conjugate combines inherent helical chirality with redox activity, which was studied in solution (both aqueous and organic phases), in layers (electropolymers), and in the solid state (a single crystal).
Abstract:
This work reports on the preparation and structural characterization of flavo[7]helicene 1 (flavin-[7]helicene conjugate), which was subsequently characterized at the molecular level in either an aqueous environment or an organic phase, at the supramolecular level in the form of polymeric layers, and also embedded in a lipidic mesophase environment to study the resulting properties of such a hybrid relative to its parent molecules. The flavin benzo[g]pteridin-2,4-dione (isoalloxazine) was selected for conjugation because of its photoactivity and reversible redox behavior. Compound 1 was prepared from 2-nitroso[6]helicene and 6-methylamino-3-methyluracil, and characterized using common structural and spectroscopic tools: circular dichroism (CD), circularly polarized luminescence (CPL) spectroscopy, cyclic voltammetry (CV), and DFT quantum calculations. In addition, a methodology that allows the loading of 1 enantiomers into an internally nanostructured lipid (1-monoolein) matrix was developed.
Robust Photocatalytic Method Using Ethylene-Bridged Flavinium Salts for the Aerobic Oxidation of Unactivated Benzylic Substrates
doi.org/10.1002/adsc.202100024
 Abstract:
Abstract:
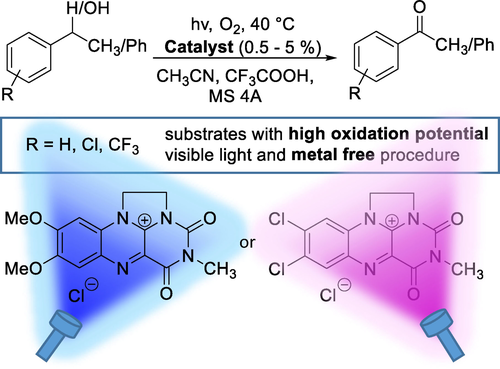
7,8-Dimethoxy-3-methyl-1,10-ethylenealloxazinium chloride (1a) was found to be a superior photooxidation catalyst among substituted ethylene-bridged flavinium salts (R=7,8-diMeO, 7,8-OCH2O-, 7,8-diMe, H, 7,8-diCl, 7-CF3 and 8-CF3). Selection was carried out based on structure vs catalytic activity and properties relationship investigations. Flavinium salt 1a proved to be robust enough for practical applications in benzylic oxidations/oxygenations, which was demonstrated using a series of substrates with high oxidation potential, i. e., 1-phenylethanol, ethylbenzene, diphenylmethane and diphenylmethanol derivatives substituted with electron-withdrawing groups (Cl or CF3). The unique capabilities of 1a can be attributed to its high photostability and participation via a relatively long-lived singlet excited state, which was confirmed using spectroscopic studies, electrochemical measurements and TD-DFT calculations. This allows the maximum use of the oxidation power of 1a, which is given by its singlet excited state reduction potential of +2.4 V. 7,8-Dichloro-3-methyl-1,10-ethylenealloxazinium chloride (1 h) can be used as an alternative photocatalyst for even more difficult substrates.
Photocatalytic Oxidative [2+2] Cycloelimination Reactions with Flavinium Salts: Mechanistic Study and Influence of the Catalyst Structure
doi.org/10.1002/cplu.202000767
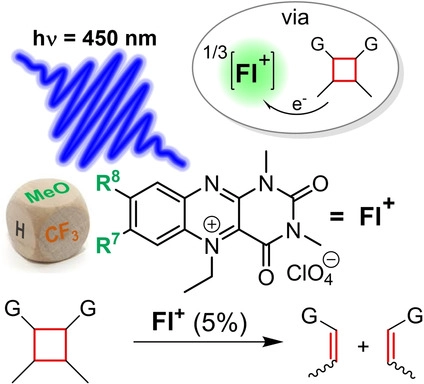
Making light work: Alloxazinium salts (flavin derivatives) are very strong oxidizing agents in their excited singlet and triplet states after absorption of visible light. When suitably substituted, these salts are relatively photostable and robust for practical applications in photoredox catalysis, as shown by the detailed mechanistic study on the [2+2] photocycloelimination reaction mediated by 7,8-dimethoxy-1,3-dimethylalloxazinium perchlorate.
Abstract:
Flavinium salts are frequently used in organocatalysis but their application in photoredox catalysis has not been systematically investigated to date. We synthesized a series of 5-ethyl-1,3-dimethylalloxazinium salts with different substituents in the positions 7 and 8 and investigated their application in light-dependent oxidative cycloelimination of cyclobutanes. Detailed mechanistic investigations with a coumarin dimer as a model substrate reveal that the reaction preferentially occurs via the triplet-born radical pair after electron transfer from the substrate to the triplet state of an alloxazinium salt. The very photostable 7,8-dimethoxy derivative is a superior catalyst with a sufficiently high oxidation power (E*=2.26 V) allowing the conversion of various cyclobutanes (with Eox up to 2.05 V) in high yields. Even compounds such as all-trans dimethyl 3,4-bis(4-methoxyphenyl)cyclobutane-1,2-dicarboxylate can be converted, whose opening requires a high activation energy due to a missing pre-activation caused by bulky adjacent substituents in cis-position.
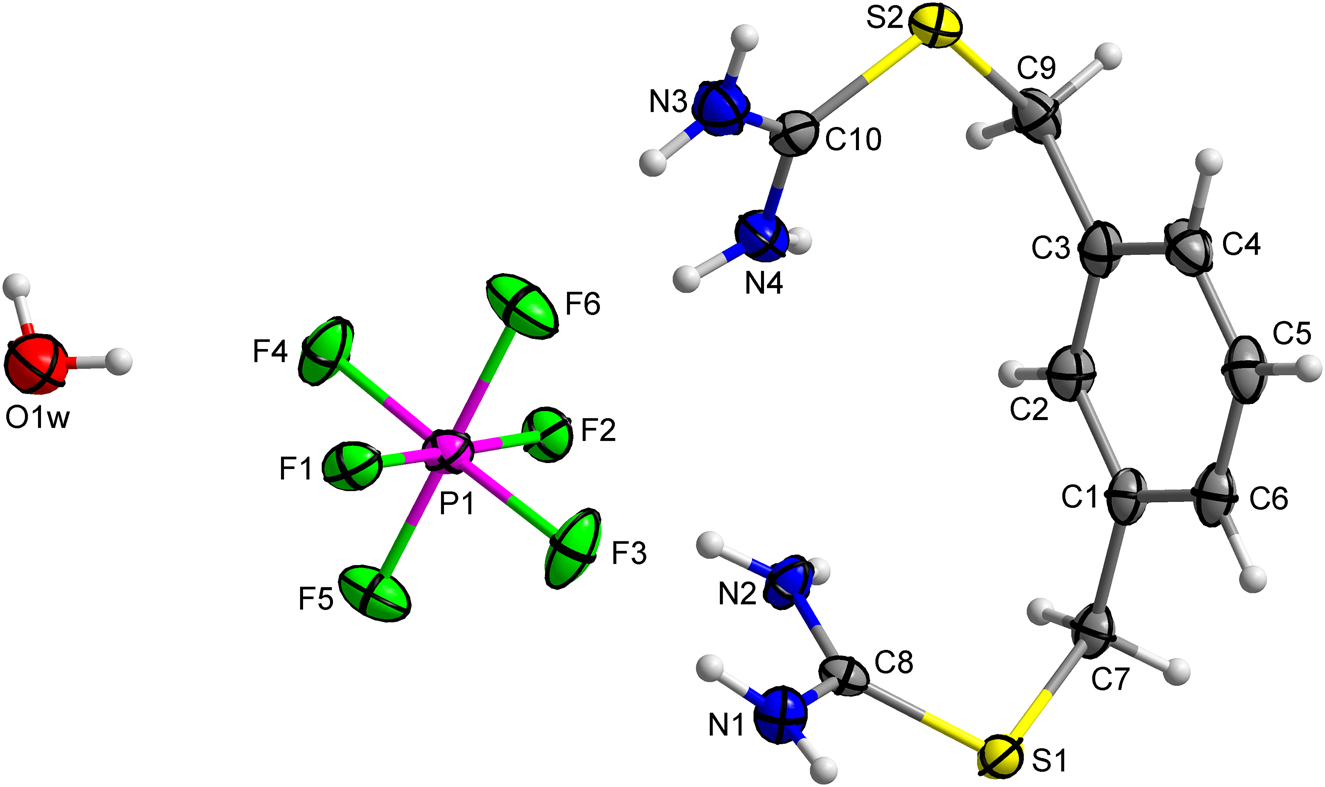
The crystal structure of 2-(4-((carbamimidoylthio)methyl)-benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
doi.org/10.1515/ncrs-2021-0417
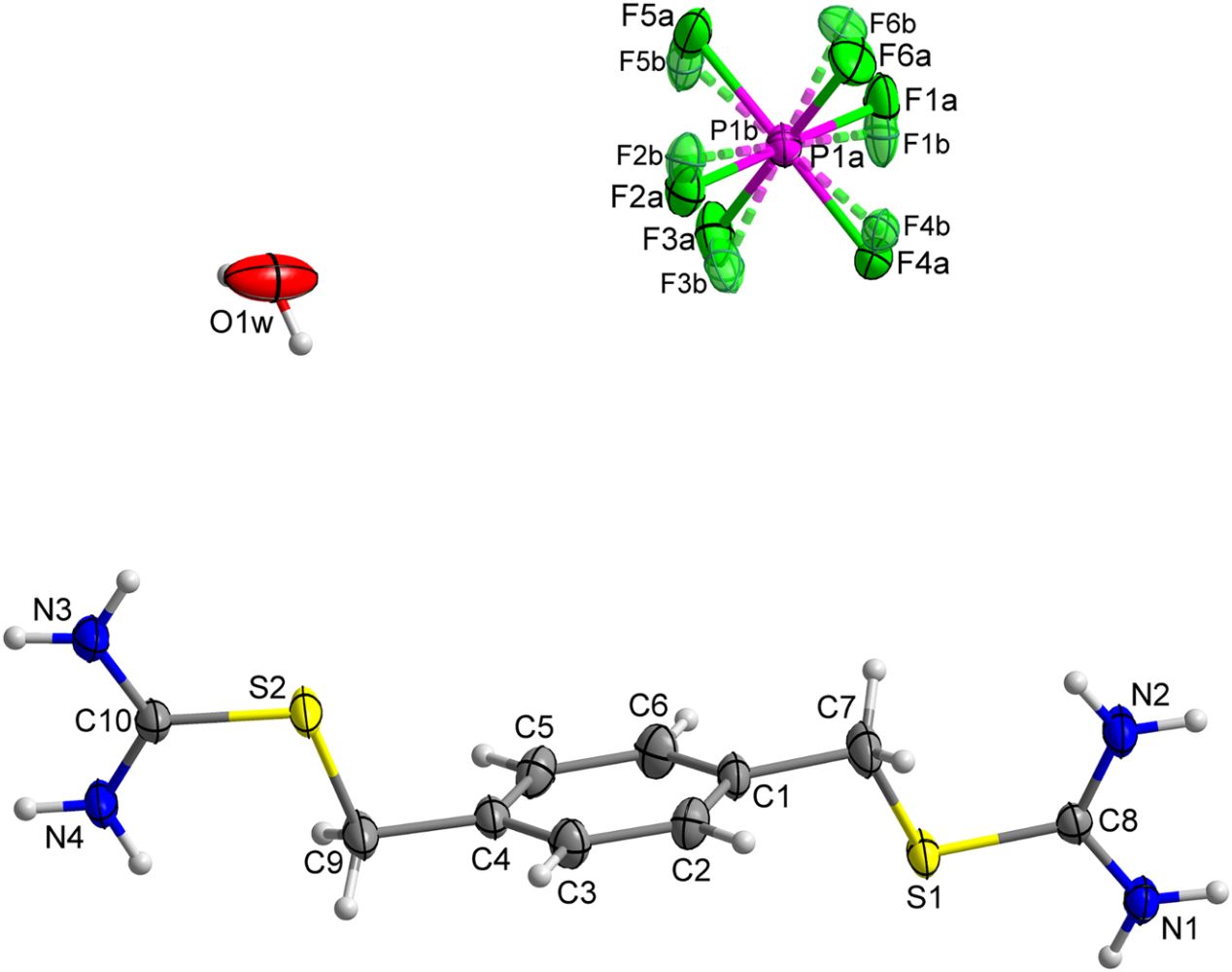
Crystal structure of 2-(3-((carbamimidoylthio)methyl)-benzyl)isothiouronium hexafluorophosphate monohydrate, C10H17F6N4OPS2
doi.org/10.1515/ncrs-2021-0418