Research topics
Photosensitive liquid crystals
Biologically active substances
Continuous flow organocatalysis
Current Special Issues guest-edited by group members
Photosensitive Liquid Crystals
https://www.mdpi.com/journal/crystals/special_issues/photosensitive_liquid_crystals
Chiral Separation by Liquid Chromatography
https://www.mdpi.com/journal/separations/special_issues/chiral_chromatography
Photosensitive liquid crystals
Light Tunable Gratings Based on Flexoelectric Effect in Photoresponsive Bent‐Core Nematics
A new photoresponsive bent‐core nematic (BCN) material, which exhibits flexoelectric domains (FDs) driven by electric field, is reported. Unexpectedly, it is found that the morphologies of FDs can be controlled by irradiation with light fields. This light tunability is ascribed to the photoisomerization effect of the azo moiety within the BCN molecules, where the ratio of trans and cis isomers changes according to the parameters of the light field, resulting in adjustable electric threshold and periodicity of FDs. Based on this principle, a prototype of controllable optical grating is assembled, whose operation can be manipulated by the wavelength or intensity of light. Due to the easy, instant, and remote operation by light, this optical, contactless tunability has a great advantage over traditional electric control in tunable photonic devices.

Jing H. et al. Adv. Opt. Mater. 2019, 7, 1801790. https://doi.org/10.1002/adom.201801790
Magnetic liquid crystals
All-organic liquid crystalline radicals with a spin unit in the outer position of a bent-core system
All-organic paramagnetic liquid crystals offer the advantage of a long-range order of liquid crystalline phases and the magnetic properties of the individual molecules. In such systems, the magnetic properties can be modified by phase transition or the application of external fields. This manuscript reports on paramagnetic all-organic bent-core liquid crystals having the radical-bearing unit (TEMPO) in the terminal position of an elongating side arm. The mesomorphic properties of the materials are ensured by the optimized molecular structure. The paramagnetic nature of the mesogenic materials is investigated by electron paramagnetic resonance, the magnetic properties of the bulk materials are studied by SQUID magnetometry. It is shown that the materials preserve their magnetic properties within the whole temperature range of liquid crystalline behaviour. Moreover, a strong correlation between spin orientation and molecular alignment within different mesophases has been observed, however, SQUID measurements do not provide evidence about spin glass formation. Unlike materials presented thus far, the position of the spin unit has a plausible effect on the formation of mesophases leading to unique polymorphism of the studied paramagnetic compounds. For two six-ring hockey-stick-like compounds, polymorphism with four different mesophases, including two B1Rev-type phases, has been found in a broad temperature range (about 20 °C each). Thus, for the first time, such behaviour is described for all-organic paramagnetic bent-core liquid crystals.
Bajzíková K. et al. J. Mater. Chem. C, 2016, 4, 11540-11547. https://doi.org/10.1039/C6TC04346A
Chiral liquid crystals
The effect of the length of terminal n-alkyl carboxylate chain on selfassembling and photosensitive properties of chiral lactic acid derivatives
A new series of photosensitive azo materials possessing a chiral alkyl lactate moiety and terminal n-alkyl carboxylate unit close to the azo group has been synthesized and studied. The length of the n-alkyl carboxylate chain has been systematically varied in order to establish the effect of the molecular structure on the self-assembling behaviour. Two series of materials possessing hexyl and dodecyl alkyl chains in the chiral part of the molecule have been studied. It has been shown that the length of both the alkyl chains strongly influences the mesomorphic behaviour, however, each chiral/achiral chain has different utility to tune the mesomorphic properties. With exception of the compound with the longest chains, all studied compounds exhibited the chiral tilted ferroelectric smectic C* phase. Based on the combination of terminal alkyl chains, chiral nematic, orthogonal smectic A*, and twist grain boundary smectic A* phases have been detected on cooling beyond the SmC* phase. The presence of the photosensitive functional N=N group in the molecular core allowed further tuning of the material properties by UV light illumination. The E-to-Z photoisomerization of the azo group and subsequent thermal back-isomerisation have been studied in solution by nuclear magnetic resonance and most importantly in the mesophases on bulk samples. We report on UV-induced isothermal switching from chiral smectic and nematic mesophases into the isotropic phase, respectively, and differences in the textures of mesophase upon restoration of the ordered liquid.
Poryvai A. et al. J. Mol. Liq. 2019, 275, 829-838. https://doi.org/10.1016/j.molliq.2018.11.058
Novel chiral ion-exchangers
Strong cation exchange-type chiral stationary phase for enantioseparation of chiral amines in subcritical fluid chromatography
A new strong cation exchange type chiral stationary phase (SCX CSP) based on a syringic acid amide derivative of trans-(R, R)-2-aminocyclohexanesulfonic acid was applied to subcritical fluid chromatography (SFC) for separation of various chiral basic drugs and their analogues. Mobile phase systems consisting of aliphatic alcohols as polar modifiers and a broad range of amines with different substitution patterns and lipophilicity were employed to evaluate the impact on the SFC retention and selectivity characteristics. The observed results point to the existence of carbonic and carbamic acid salts formed as a consequence of reactions occurring between carbon dioxide, the alcoholic modifiers and the amine species present in the sub/supercritical fluid medium, respectively. Evidence is provided that these species are essential for affecting ion exchange between the strongly acidic chiral selector units and the basic analytes, following the well-established stoichiometric displacement mechanisms. Specific trends were observed when different types of amines were used as basic additives. While ammonia gave rise to the formation of the most strongly eluting carbonic and carbamic salt species, simple tertiary amines consistently provided superior levels of enantioselectivity. Furthermore, trends in the chiral SFC separation characteristics were investigated by the systematic variation of the modifier content and temperature. Different effects of additives are interpreted in terms of changes in the relative concentration of the transient ionic species contributing to analyte elution, with ammonia-derived carbamic salts being depleted at elevated temperatures by decomposition. Additionally, in an effort to optimize SFC enantiomer separation conditions for selected analytes, the impact of the type of the organic modifier, temperature, flow rate and active back pressure were also investigated.
Wolrab D. et al. J. Chromatogr. A 2013, 1289, 94-104. https://doi.org/10.1016/j.chroma.2013.03.018
Chiral separation
Consequences of transition from liquid chromatography to supercritical fluid chromatography on the overall performance of a chiral zwitterionic ion-exchanger
Major differences in the chromatographic performance of a zwitterion ion-exchange type (ZWIX) chiral stationary phase (CSP) in supercritical fluid chromatography (SFC) and high-performance liquid chromatography (HPLC) have been observed. To explain these differences, transition from HPLC to SFC conditions has been performed. The amount of a protic organic modifier in supercritical carbon dioxide (scCO2) was stepwise increased and the effect of this change studied using acidic, basic and ampholytic analytes. At the same time, the effect of various basic additives to the mobile phase and transient acidic buffer species, formed by the reaction of scCO2 with the organic modifier and additives, was assessed. Evidence is provided that a transient acid together with the intrinsic counter-ions present in the ZWIX selector structure drive the elution of analytes even when no buffer is employed. We show that the tested analytes can be enantioseparated under both SFC and HPLC conditions; the best conditions for the resolution of ampholytes are in the so-called enhanced-fluidity mobile phase region. As a consequence, subcritical fluid and enhanced-fluidity mobile phase regions seem to be chromatographic modes with a high potential for operating ZWIX CSPs.
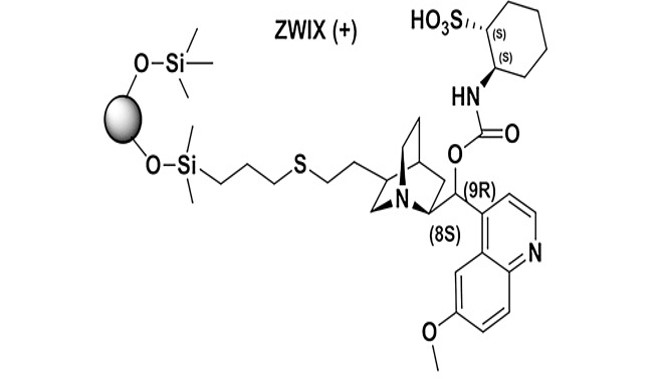
Wolrab D. et al. J. Chromatogr. A 2017, 1517, 165-175. https://doi.org/10.1016/j.chroma.2017.08.022
Biologically active substances
Synthesis, absolute configuration and in vitro cytotoxicity of deschloroketamine enantiomers: rediscovered and abused dissociative anaesthetic
In this study, we aim to determine differences in cytotoxicity of racemic deschloroketamine and its enantiomers. The synthesized racemate of this recently rediscovered and abused dissociative anaesthetic was resolved by chiral HPLC and the absolute configuration of the enantiomers was assigned using a combination of circular dichroism methods and single-crystal X-ray. Not only the absolute configuration, but also the most preferred conformers present in the crystal were successfully determined by electron and vibrational circular dichroism supported by ab initio calculations, and confirmed by X-ray. The in vitro cytotoxicity of racemic deschloroketamine and its enantiomers was determined for nine different types of cell lines. Generally, (S)-deschloroketamine exhibited higher cytotoxicity in the majority of cases. For human embryonic kidney cells (HEK 293T), the (S)-enantiomer reached the IC50 below 1 mM concentration and, in consequence, proved to be twice as potent as the (R)-enantiomer. On the other hand, live-cell fluorescence microscopy imaging at sub-IC50 concentrations provided evidence for only a minor effect of deschloroketamine racemate and enantiomers on endoplasmic reticulum stress and mitochondria morphology in neuroblastoma cells SH-SY5Y.
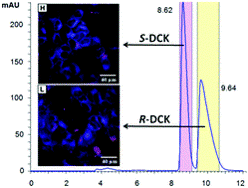
Jurásek B. et al. New J. Chem., 2018, 42, 19360-19368. https://doi.org/10.1039/C8NJ03107J
Continuous flow organocatalysis
Silica gel-immobilized multidisciplinary materials applicable in stereoselective organocatalysis and HPLC separation
In this pilot study, we present novel bifunctional silica gel-immobilized materials applicable as heterogeneous organocatalysts and stationary phases in HPLC. The materials provided high stereoselectivity in both batch and continuous flow catalysis of a model Michael addition (cyclohexanone to (E)-β-nitrostyrene). In the batch reaction, the catalysts proved their sustainable catalytic activity over five consecutive recycling experiments. Under continuous flow reaction conditions, the catalytic activity was found to be superior to the batch reaction, and moreover, the same immobilized materials were utilized as stationary phases in HPLC showing very good chemoselective separation of model acidic analytes.
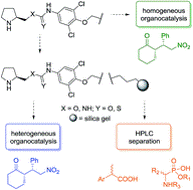
Tůma J. et al. RSC Adv., 2018, 8, 1174-1181. https://doi.org/10.1039/C7RA12658A
