Publikace našich autorů
2026
- Sponar R., Liška A., Cibulka R.:
Electrochemistry Facilitates the hemoselectivity of Benzylic Alcohol Oxidations Mediated by Flavin-Based Photoredox Catalysis
Org. Lett. 2026, accepted, DOI: 10.1021/acs.orglett.5c04603 - Kvíčala J.; Rybáčková, M.:
Hypervalent fluorinated organosilicon compounds: synthesis, structure and applications
Coord. Chem. Rev. 2026, 551, 217489, https://doi.org/10.1016/j.ccr.2025.217489
2025
- Dundálková, P.; Rybáčková, M.; Cvačka, J.; Čejka, J.; Kvíčala J.:
Hoveyda–Grubbs 1st and 2nd Generation Precatalysts Modified in the Alkoxybenzylidene Part With Long (Fluorous) Chains: Catalytic Activity and Stability
Eur. J. Org. Chem. 2025; e202500654, https://doi.org/10.1002/ejoc.202500654 - Weisheitelová I., Varma N., Šimková L., Chudoba J., Pavlovska T., Gulaczyk I., Burdziński G., Ludvík J., Sikorski M., Cibulka R.:
Deazaalloxazines – Flavin Derivatives That Provide Reductive Photoredox Catalysis with Inert Substrates,
Chem. Eur. J. 2025, e02897, accepted. DOI: 10.1002/chem.202502897. - Tolba A. H., El-Zohry A., Khan J. I., Svobodová E., Chudoba J., Klíma J., Lušpai K., Pižl M., Šturala J., Cibulka R.:
Redox-innocent scandium(III) as the sole catalyst in visible light photooxidations
Nat. Commun. 2025, 16, 7851. - Rückel J., Pavlovska T., Gawrona M., Cibulka R., Wolf R.:
Deazaflavin-Catalyzed Arylation of White Phosphorus with Aryl Bromides and Chlorides.
Adv. Synth. Catal. 2025, 367, e70005. DOI 10.1002/adsc.202500435 - Prukała D., Zubova E., Svobodová E., Šimková L., Varma N., Chudoba J., Ludvík J., Burdzinski G., Gulaczyk G., Sikorski M., Cibulka R.:
Introduction of flavin anions into photoredox catalysis: Acid-base equilibria of lumichrome allows photoreductions with an anion of an elusive 10-unsubstituted isoalloxazine.
Chem. Sci. 2025, 16, 11255 - 11263. DOI: 10.1039/D5SC01630D
- Šetek, M.; Guerra, V. L. P.; Robson, H.; Geleverya, A. O.; Maxa, O.; Lamancová, A.; Eigner, V.; Nachtigallová, D.; Tarábek, J.; Kovaříček, P.:
Closed-to-open-shell ground state photoswitching of thienyl-based acylhydrazones.
J. Mater. Chem. C, 2025, Advance Article, https://doi.org/10.1039/D5TC00826C - Ferenczei, J.; Blahout, V.; Dvořáková, H.; Brancale, A.; Cuřínová, P.; Labíková, M.; Kohout, M.; Setnička, V.; Perlíková, P.:
Stereoanalysis of the antiparasitic natural product callunene and its synthetic intermediates.
J. Nat. Prod. 2025, 88, 723–731. DOI: 10.1021/acs.jnatprod.4c01424 - Liang, F.; Jing, H.; Fu, J.; Xiang, Y.; Salamon, P.; Éber, N.; Buka, A.; Kohout, M.; Li, J.; Chen, J.:
Controlling diffracted beam-polarization by patterned optical-field in a photosensitive chiral nematic.
J. Mol. Liq. 2025, 420, 126814. https://doi.org/10.1016/j.molliq.2024.126814 - Čížek, J.; Jandová, V.; Stanovský, P.; Hovorka, Š.; Yalcinkaya, F.; Kohout, M.; Izák, P.:
Chiral membranes prepared by ionic interactions between sulfobutylether-b-cyclodextrin and anion-exchange membranes.
J. Membr. Sci. 2025, 717, 123592. https://doi.org/10.1016/j.memsci.2024.123592 - Dobšíková, K.; Kohout, M.; Setnička, V.:
Chiral separation and spectroscopic characterization of mefloquine analogues.
Spectrochim. Acta A 2025, 324, 124940. https://doi.org/10.1016/j.saa.2024.124940 - Burešová Z., Grygarová M., Prokopová E., Klikar M., Pytela O., Váňa J., Fahim M. A. M., Kanyashree J., Zubova E., Bartáček J., Tydlitát J., Růžičková Z., Cibulka R., Schanze K. S., Bureš F.:
Divergent photoreduction of nitroaromatic compounds catalysed by dithienoquinoxaline
J. Catal. 2025, 445, 116033. DOI: 10.1016/j.jcat.2025.116033 - Koudelka, J., Tobrman, T.:
Three-component synthesis of β-sulfonyl enamines and dienamines enabled by silver(i) acetate
RSC Adv. 2025, 15, 3602-3606, DOI: https://doi.org/10.1039/D4RA08480B - Pavlovska T., Havlík M., Labíková M., Cibulka R.:
Biomimetic Orthogonal Removal of Arylacyl Protecting Groupsfrom the C-Terminus of Substituted Amino Acids andOligopeptides via Flavin N(5)-iminium Covalent Adducts.
Adv. Synth. Catal. 2025, 367, e202401556. DOI: 10.1002/adsc.202401556 - Ledvinka, J., Rota Sperti, F., Paragi, G., Pirrotta, M., Chéron, N., Valverde, I. E., Menova, P., Monchaud D.:
Fluorescence Detection of DNA/RNA G-Quadruplexes (G4s) by Twice-asSmart Ligands
ChemMedChem 2025, e202400829, doi.org/10.1002/cmdc.202400829
2024
- Trojan, M.; Hroch, A.; Gruden, E.; Cvačka, J.; Čejka, J.; Tavčar, G.; Rybáčková, M.; Kvíčala, J.:
Modified aryldifluorophenylsilicates with improved activity and selectivity in nucleophilic fluorination of secondary substrates
RSC Adv., 2024, 14, 22326, https://doi.org/10.1039/D4RA04332D - Trojan, M.; Kučnirová, K.; Bouzková, Š.; Cvačka, J.; Čejka, J.; Tavčar, G.; Rybáčková, M.; Kvíčala, J.:
Quaternary ammonium fluorides and difluorosilicates as nucleophilic fluorination reagents
Org. Biomol. Chem., 2024, 22, 1047, https://doi.org/10.1039/D3OB01875J - Tetour, D.; Novotná, M.; Tatýrek, J.; Máková, V.; Stuchlík, M.; Hobbs, C.; Řezanka, M.; Müllerová, M.; Setnička, V.; Dobšíková, K.; Hodačová, J.:
Preparations of spherical nanoparticles of chiral Cinchona alkaloid-based silsesquioxanes and their use in heterogeneous catalysis of enantioselective reactions.
Nanoscale 2024, 16, No. 13, 6696-6707, https://doi.org/10.1039/d3nr06234a - Rosado, A.; Borrás, A.; Sánchez-Soto, M.; Labíková, M.; Hettegger, H.; Ramírez-Jimenéz, R. A.; Rojo, L.; García-Fernández, L.; Aguilar, M. R.; Liebner, F.; López-Periago, A. M.; Ayllón, J. A.; Domingo, C.:
BioMOF@cellulose Glycerogel Scaffold with Multifold Bioactivity: Perspective in Bone Tissue Repair.
Gels 2024, 10, 631. https://doi.org/ 10.3390/gels10100631 - Fuxová, H.; Labíková, M.; Ivanovská, A.; Eliášová, P.; Kubů, M.; Hovorka, Š.; Přibyl, M.; Čížek, J.; Bartůněk, V.; Kohout, M.; Izák, P.:
Zeolite-based chiral ion-exchangers for chromatographic enantioseparations and potential applications in membrane separation processes.
Talanta 2024, 278, 126419. https://doi.org/10.1016/j.talanta.2024.126419 - Boháček, V.; Erbenová, T.; Malina, J. D.; Kloubcová, M.; Šmahel, M.; Eigner, V.; Tůma, J.:
Spectroscopic and photochemical evaluation of stereochemically biased 3‘-substituted spiropyran photoswitches.
RSC Adv. 2024, 14, 37370. https://doi.org/10.1039/D4RA07750D - Varfaj, I.; Labíková, M.; Sardella, R.; Hettegger, H.; Lindner, W.; Kohout, M.; Carotti, A.:
A journey in unraveling the enantiorecognition mechanism of 3,5-dinitrobenzoyl-amino acids with two Cinchona alkaloid-based chiral stationary phases: The power of molecular dynamic simulations.
Anal. Chim. Acta 2024, 1314, 342791. https://doi.org/10.1016/j.aca.2024.342791 - Tichotová, M.; Landovský, T.; Lang, J.; Jeziorowski, S.; Schmidts, V.; Kohout, M.; Babor, M.; Lhoták, P.; Thiele, C.M.; Dvořáková, H.:
Enantiodiscrimination of Inherently Chiral Thiacalixarenes by Residual Dipolar Couplings.
J. Org. Chem. 2024, 89, 9711-9720. - Asnin, L.D.; Ziganshina, D.I.; Klimova, Y.A.; Reshetova, E.N.; Tůma, J.; Kohout, M.:
Chiral zwitterionic stationary phases based on Cinchona alkaloids and dipeptides: Application in chiral separation of dipeptides under reversed phase conditions.
J. Chromatogr. A 2024, 1726, 464966. https://doi.org/10.1016/j.chroma.2024.464966 - Paškanová, N.; Hlavatá, P.; Jurásek, B.; Kuchař, M.; Kohout, M.:
Tuning parameters of single quadrupole mass detector hyphenated to supercritical fluid chromatography for enantioseparation of synthetic cathinones.
J. Chromatogr. Open 2024, 5, 100139. https://doi.org/10.1016/j.jcoa.2024.100139 - Miliutina, E.; Shilenko, V.; Burtsev, V.; Petkevich, A.; Elashnikov, R.; Buravets, V.; Kolská, Z.; Kohout, M.; Švorčík, V.; Lyutakov, O.:
Homochiral Optical Fibers Coated with Nanoscale Films of Metal-Organic Frameworks and Double-Plasmonic Ag and Au for In Situ Enantioselective Detection.
ACS Appl. Nano Mater. 2024, 7, 9210-9217. https://doi.org/10.1021/acsanm.4c00624 - Luguo, H.A.O.; Liang, F.; Jing, H.; Xiang, Y.; Salamon, P.; Éber, N.; Buka, A.; Kohout, M.; Chen, J.; Pei, Y.:
Control of light polarization by optically-induced-chirality in photosensitive nematic fluids.
Opt. Express 2024, 32, 13965-13977. https://doi.org/10.1364/OE.522820 - Labíková, M.; Svoboda, J.; Tůma, J.; Lindner, W.; Kohout, M.:
Chiral recognition without π−π-interactions: Highly efficient chiral strong cation exchangers lacking an aromatic unit in the molecular structure.
J. Chromatogr. A 2024, 1719, 464729. https://doi.org/10.1016/j.chroma.2024.464729 - Kuncová, A.; Svoboda, J.; Tůma, J.; Asnin, L.; Schug, K.; Kohout, M.:
Chiral zwitterionic stationary phases based on Cinchona alkaloids and dipeptides – design, synthesis and application in chiral separation.
J. Chromatogr. A 2024, 1717, 464664. https://doi.org/10.1016/j.chroma.2024.464664 - Paškan, M.; Dobšíková, K.; Kuchař, M.; Setnička, V.; Kohout, M.:
Synthesis and absolute configuration of cyclic synthetic cathinones derived from α-tetralone.
Chirality 2024, 36, e23646. https://doi.org/10.1002/chir.23646 - Svoboda, J.; Kozmík, V.; Bajzíková, K.; Kohout, M.; Novotná, V.; Podoliak, N.; Pociecha, D.; Gorecka, E.:
Competing synclinic and anticlinic interactions in smectic phases of bent-core mesogens.
J. Mater. Chem. C 2024, 12, 10903-10909. https://doi.org/10.1039/D4TC01695E - Bečvář, P.; Krystianiak, A.; Ganesh Moorthy, S.; Jansová, B.; Kohout, M.; Meunier-Prest, R.; Bouvet, M.:
Electrosynthesized fluorinated polybithiophenes for ammonia sensing.
Mater. Chem. Front. 2024, 8, 2666-2680. https://doi.org/10.1039/D4QM00323C - Chu, Y.; Xiang, Y.; Zhang, W.; Hao, L.; Jiang, B.; Kai, Ch.; Gao, Y.; Yongchuan, H.; Kohout, M.:
Patch antenna adjustable in frequency based on polymer dispersed liquid crystal.
Chin. J. Liq. Cryst. Disp. 2024, 39, 17-24. - Ramírez-Cortés F., Ménová P.:
Hepatocyte targeting via the asialoglycoprotein receptor.
RSC Med. Chem. 2024, Advance Article. DOI: https://doi.org/10.1039/D4MD00652F. - Obertík R., Ludvíková L., Chudoba J., Cibulka R.:
Chemoselective Anaerobic Dehydrogenation of Alcohols By Using Visible Light and a Positively Charged Deazaflavin Catalyst Regenerated by Acetonitrile Solvent.
ChemCatChem 2024, accepted. DOI: 10.1002/cctc.202401795. - Niziński S., Varma N., Sikorski M., Tobrman T., Svobodová E., Cibulka R., Rode M. F., Burdzinski G.:
Fast singlet excited-state deactivation pathway of flavin with a trimethoxyphenyl derivative.
Sci Rep 2024, 14, 24375. DOI: 10.1038/s41598-024-75239-x. - Weisheitelová I., Cibulka R., Sikorski M., Pavlovska T.:
A facile three-component route to powerful 5-aryldeazaalloxazine photocatalysts.
Beilstein J. Org. Chem. 2024, 20, 1831–1838. DOI: 10.3762/bjoc.20.161.
- Venkatraman R. K., Hassan Tolba A., Solling T., Cibulka R., El-Zohry A.:
Ultrafast Events of Photoexcited Iron(III)chloride for Activation of Benzylic C—H Bonds.
J. Phys. Chem. Lett. 2024, 15, 6202−6208, DOI: 10.1021/acs.jpclett.4c011 - Weisheitelová I., Varma N., Chudoba J., Burdziński G., Sikorski M., Cibulka R.:
Catalyst-free aerobic photooxidation of sensitive benzylic alcohols with chemoselectivity controlled by DMSO solvent.
Green Chem. 2024, 26, 4880-4887, https://doi.org/10.1039/D4GC00087K - Burešová Z., Gobeze H. B., Grygarová M., Pytela O., Klikar M., Obertík R., Cibulka R., Islam T., Schanze K. S., Bureš F.:
Dicyanopyrazine Photoredox Catalysts: Correlation of Efficiency with Photophysics and Electronic Structure.
J. Catal. 2024, 430, 115348, DOI: 10.1016/j.jcat.2024.115348 - Salvadori, K.; Churý, M.; Budka, J.; Harvalík, J.; Matějka, P.; Šimková, L.; Lhoták, P.:
Chemoselective Electrochemical Cleavage of Sulfonimides as a Direct Way to Sulfonamides.
J. Org. Chem. 2024, 89, 1425–1437, https://doi.org/10.1021/acs.joc.3c01932
2023
- Kučnirová, K.; Kvíčala, J.; Svoboda, M.; Cvačka, J.; Čejka, J.; Rybáčková, M.:
Non-Symmetrical Tetrafluoroalkadienes Synthesized by ROCM of 3,3,4,4-Tetrafluorocyclobutene
Chem. Eur. J. 2023, 29, e202300435, https://doi.org/10.1002/chem.202300435 - Jurášek, M.; Kratochvíl, B.; Kohout, M.; Švec, F.; Drašar, P.:
On the separation and strengthening of the image behind the mirror (Deracemization and chirality amplification).
Chem. listy 2023, 117, 671-676. https://doi.org/10.54779/chl20230671 - Valderhaug, S.; Paškanová, N.; Tůma, J.; Herciková, J.; Eigner, V.; Liu, H.; Gorovoy, A.; Johansen, J. E.; Gautun, O. R.:
Synthesis, identification, chiral separation and crystal structure of (3R, 4R, 7S, 8S)-3,4,7,8-tetrachlorodecane and its stereoisomers.
Heliyon 2023, 9, e16987. https://doi.org/j.heliyon.2023.e16987 - Zubova, E., Pokluda A., Dvořáková, H., Krupička M., Cibulka R.:
Exploring the Reactivity of Flavins with Nucleophiles Using a Theoretical and Experimental Approach.
ChemPlusChem 2023, Accepted. DOI:10.1002/cplu.202300547. - Pavlovska T., Weisheitelová I., Pramthaisong C., Sikorski M., Jahn U., Cibulka R.:
Primary and Secondary Amines by Flavin‐Photocatalyzed Consecutive Desulfonylation and Dealkylation of Sulfonamides.
Adv. Synth. Catal. 2023, 365, 4662-4671. DOI:10.1002/adsc.202300843. - Golczak A., Prukała D., Gierszewski M., Cherkas V., Kwiatek D., Kubiak A., Varma N., Pedziński T., Murphree S., Cibulka R., Mrówczińska L., Kolanovski J. L., Sikorski M.:
Tetramethylalloxazines as efficient singlet oxygen photosensitizers and potential redox‑sensitive agents, Scientific Reports 2023, 13, 13426. DOI: 10.1038/s41598-023-40536-4. - Pokluda A., Zubova, E., Chudoba J., Krupička M., Cibulka R.:
Catalytic Artificial Nitroalkane Oxidases – a Way Towards Organocatalytic Umpolung.
Org. Biomol. Chem. 2023, 2768 – 2774. DOI: 10.1039/d3ob00101f. - Jurásek, B.; Fagan, P.; Dolenský, B.; Paškanová, N.; Dobšíková, K.; Raich, I.; Jurok, R.; Setnička, V.; Kohout, M.; Čejka, J.; Kuchař, M.:
A structural spectroscopic study of dissociative anaesthetic methoxphenidine. New J. Chem. 2023, 47, 4543.
https://doi.org/10.1039/d2nj06126k - Dobšíková, K.; Javorská, Ž.; Paškan, M.; Spálovská, D.; Trembuláková, P.; Herciková, J.; Kuchař, M.; Kozmík, V.; Kohout, M.; Setnička, V.:
Enantioseparation and a comprehensive spectroscopic analysis of novel synthetic cathinones laterally substituted with a trifluoromethyl group. Spectrochim. Acta, Part A, 2023, 291, 122320.
https://doi.org/10.1016/j.saa.2023.122320
2022
- Cortés F.R., Eigner V., Cuřínová P., Himl M.:
Structurally Forced Ion Binding Affinity: Tetraurea-Based Macrocycle as a Receptor for Ion Pairs
Eur. J. Org. Chem. 2022, e202200, DOI: 10.1002/ejoc.202200422 - Obertík R., Chudoba J., Šturala J., Tarábek J., Ludvíková L., Slanina T., König B., Cibulka R.:
Highly Chemoselective Catalytic Photooxidations Using Solvent as a Sacrificial Electron Acceptor
Chem. Eur. J. 2022, accepted as VIP, https://doi.org/10.1002/chem.202202487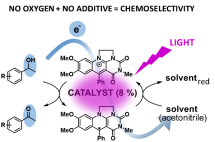
- Liška, A.; Řezanková, M.; Klíma, J.; Urban, J.; Budka, J.; Ludvík, J.:
Electrochemical, EPR, and quantum chemical study of reductive cleavage of cone-Calix[4]arene nosylates – New electrosynthetic approach
Electrochem. Sci. Adv. 2022, e2100221, DOI: org/10.1002/elsa.202100221 - Zhang, H.; Daněk, O.; Makarov, D.; Rádl, S.; Kim, D.; Ledvinka, J.; Vychodilová, K.; Hlaváč, J.; Lefèbre, J; Denis, M.; Rademacher, C. and Ménová, P.:
Drug-like Inhibitors of DC-SIGN Based on a Quinolone Scaffold
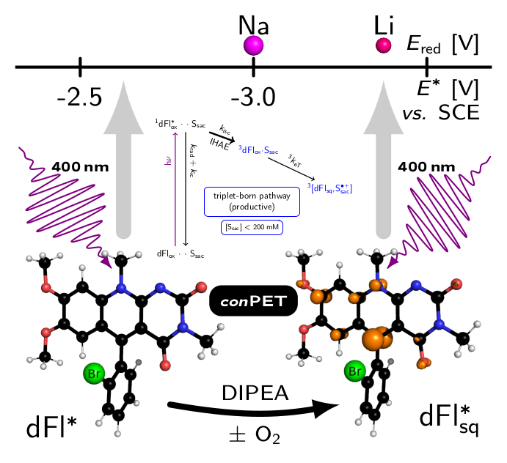
ACS Med. Chem. Lett. 2022, accepted. DOI: 10.1021/acsmedchemlett.2c00067 - Pavlovska T., Král Lesný D., Svobodová E., Hoskovcová I., Archipowa N., Kutta R. J., Cibulka R.:
Tuning Deazaflavins Towards Highly Potent Reducing Photocatalysts Guided by Mechanistic Understanding - Enhancement of the Key Step by the Internal Heavy Atom Effect. Chem. Eur. J. 2022, 2022, 28, e20220076. Hot Paper. DOI: 10.1002/chem.202200768
- Linhart, I.:
Toxikologie: Interakce škodlivých látek s živými organismy, jejich mechanismy, projevy a důsledky
VŠCHT Praha 2022 (3. vydání), ISBN 978-80-7592-103-1
- Linhart, I.; Himl, M.; Urban, V.; Mráz, J.:
Syntheses of methylcarbamoylated amino acids using synthetic equivalents of methyl isocyanate.
Synth. Commun. 2022, 52, 622-628. DOI: 10.1080/00397911.2022.2042563 - Paškan, M.; Rimpelová, S.; Svobodová Pavlíčková, V.; Spálovská, D.; Setnička, V.; Kuchař, M.; Kohout, M.:
4-Isobutylmethcathinone—A novel synthetic cathinone with high in vitro cytotoxicity and strong receptor binding preference of enantiomers. Pharmaceuticals 2022, 15, 1495.
https://doi.org/10.3390/ph15121495 - Hao, L.; Jing, H.; Xiang, Y.; Iljin, A.; Wang, Y.; Li, H.; Li, Q.; Peng, J.; Kohout, M.:
Transient optically induced grating and underlying transport process in bent-core nematics. J. Appl. Phys. 2022, 132, 065108.
https://doi.org/10.1063/5.0096106 - Sysel, P.; Hovorka, Š.; Kohout, M.; Holakovský, R.; Žádný, J.; Čížek, J.; Izák, P.:
Optically active polyimides with different thermal histories of their preparation. Chirality 2022, 34, 1151-1161.
https://doi.org/10.1002/chir.23476 - Asnin, L.; Herciková, J.; Lindner, W.; Klimova, Y.; Ziganshina, D.; Reshetova, E.; Kohout, M.:
Chiral separation of dipeptides on Cinchona-based zwitterionic chiral stationary phases under buffer-free reversed-phase conditions. Chirality 2022, 34, 1065-1077.
https://doi.org/10.1002/chir.23471 - Malinčík, J.; Kohout, M.; Svoboda, J.; Stulov, S.; Pociecha, D.; Böhmová, Z.; Novotná, V. Photochromic spiropyran-based liquid crystals. J. Mol. Liq. 2022, 346, 117842.
https://doi.org/10.1016/j.molliq.2021.117842
2021
- Pokluda A., Anwar Z., Boguschová V., Anusiewicz I., Skurski P., Sikorski M., Cibulka R.:
Robust photocatalytic method using ethylene-bridged flavinium salts for the aerobic oxidation of unactivated benzylic substrates
Adv. Synth. Catal. 2021, 363, 4371-4379. VIP, DOI: 10.1002/adsc.202100024.
- Jakubec M., Novák D., Zatloukalová M., Sýkora J., Císařová I., Cibulka R., Favereau L., Crassous J., Bilewicz R., Hrbáč J., Storch J., Žádný J., Vacek J.:
Flavin-Helicene Amphiphilic Hybrids: Synthesis, Characterization, and Preparation of Surface-Supported Films
ChemPlusChem 2021, 86, 982-990. DOI: 10.1002/cplu.202100092
- Tolba A. H., Krupička M., Chudoba J., Cibulka R.:
mide bond formation via aerobic photooxidative coupling of aldehydes with amines catalyzed by a riboflavin derivative
Org. Lett. 2021, 23, 6825-6830. DOI: 10.1021/acs.orglett.1c02391
- Tetour, D.; Novotná, M.; Hodačová, J.:
Enantioselective Henry Reaction Catalyzed by Copper(II) Complex of Bis(trans-cyclohexane-1,2-diamine)-Based Ligand.
Catalysts 2021, 11, 41. http:\\dx.doi.org/10.3390/catal11010041 - Cadart, T.; Nečas, D.; Kaiser, R. P.; Favereau, L.; Císařová, I.; Gyepes, R.; Hodačová, J.; Kalíková, K.; Bednárová, L.; Crassous, J.; Kotora, M.:
Rhodium-Catalyzed Enantioselective Synthesis of Highly Fluorescent and CPL Active Dispiroindeno[2,1‐c]fluorenes.
Chem. Eur. J. 2021, 27, 11279-11284.https://doi.org/10.1002/chem.202100759 - Tetour, D.; Paška, T.; Máková, V.; Nikendey Holubová, B.; Karpíšková, J.; Řezanka, M.; Brus, J.; Hodačová, J.:
Cinchonine-based organosilica materials as heterogeneous catalysts of enantioselective alkene dihydroxylation.
J. Catal. 2021, 404, 493-500. https://doi.org/10.1016/j.jcat.2021.10.015 - Kortus, D.; Krizova, K.; Dvorakova, H.; Eigner, V.; Lhotak, P.:
Synthesis of 2,8-dithiacalix[4]arene based on fragment condensation
Tetrahedron Lett. 2021, 69, 152924 DOI:10.1016/j.tetlet.2021.152924 - Kortus, D.; Eigner, V.; Lhotak, P.:
Regio- and stereoselectivity of spirodienone formation in 2,14-dithiacalix[4]arene
New J. Chem. 2021, 45, 8563-8571 DOI:10.1039/d1nj01257f - Kortus, D.; Kundrat, O.; Cejka, J.; Dvorakova, H.; Lhotak, P.:
Chemistry of 2,14-Dithiacalix[4]arene: Searching for the Missing Fifth Conformer
J. Org. Chem. 2021 DOI:10.1021/acs.joc.1c01173 - Curinova, P.; Winkler, M.; Krupkova, A.; Cisarova, I.; Budka, J.; Wun, C.N.; Blechta, V.; Maly, M.; Stastna-Cervenkova, L.; Sykora, J.; Strasak, T.:
Transport of Anions across the Dialytic Membrane Induced by Complexation toward Dendritic ReceptorsACS Omega 2021, 23, 15514-15522 DOI:10.1021/acsomega.1c02142
- Hartman T., Reisnerová M., Chudoba J., Svobodová E., Archipowa N., Kutta R. J., Cibulka R.:
Photocatalytic Oxidative [2+2] Cycloelimination Reactions with Flavinium Salts: Mechanistic Study and Influence of the Catalyst Structure
ChemPlusChem 2021, 86, 373–386. DOI: 10.1002/cplu.202000767
COVER:
- Edlová, T.; Čubiňák, M.; Tobrman, T.:
Cross-Coupling Reactions of Double or Triple Electrophilic Templates for Alkene Synthesis.
Synthesis 2021, 53, 255-266. DOI: 10.1055/s-0040-1707270 - Tobrman, T.:
Synthesis of 2-Substituted Cyclobutanones by a Suzuki Reaction and Dephosphorylation Sequence
Eur. J. Org. Chem. 2021, 3260-3269 DOI:10.1002/ejoc.202100464 - Tobrman, T.:
Substrate-Controlled Regioselective Bromination of 1,2-Disubstituted Cyclobutenes: An Application in the Synthesis of 2,3-Disubstituted Cyclobutenones
J. Org. Chem. 2021, 86, 5820-5831 DOI:10.1021/acs.joc.1c00261 - ; Tobrman, T.; J
- Skopalová H., Kozmík V., Šmahel M., Svoboda J., Pacherová O., Kohout M., Novotná, V.:
Mesomorphic properties of non-symmetric bent-core liquid crystals with a lateral substituent in the apex position
Liq. Cryst. 2021, 48, 1010-1024. https://doi.org/10.1080/02678292.2020.1836567 - Cigl M., Hampl F., Svoboda J., Podoliak N., Stulov S., Kohout M., Novotná V.:
Laterally substituted biphenyl benzoates – synthesis and mesomorphic properties
Liq. Cryst. 2021, 48, 526-536. https://doi.org/10.1080/02678292.2020.1794069 - Spálovská D., Paškan M., Jurásek B., Kuchař M., Kohout M., Setnička V.:
Structural spectroscopic study of enantiomerically pure synthetic cathinones and their major metabolites
New J. Chem. 2021, 45, 850. https://doi.org/10.1039/D0NJ05065B - Wolrab D., Frühauf P., Kolderová N., Kohout M.:
Strong cation- and zwitterion-exchange-type mixed-mode stationary phases for separation of pharmaceuticals and biogenic amines in different chromatographic modes
J. Chromatogr. A 2021, 1635, 461751. https://doi.org/10.1016/j.chroma.2020.461751 - Linhart I., Hanzlíková I., Mráz J., Dušková Š., Tvrdíková M., Vachová H.:
Novel aminoarylcysteine adducts in globin of rats dosed with naphthylamine and nitronaphthalene isomers.
Arch. Toxicol. 2021, 95, 79-89. doi: 10.1007/s00204-020-02907-y - Marques M.M.; Beland F.A.; Lachenmeier D.W.; Phillips D.H.; Chung F.L.; Dorman D.C.; Elmore S.E.; Hammond S.K.; Krstev S.K.; Linhart I.; Long A.S.; Mandrioli D.; Ogawa K.; Pappas J.J.; Parra Morte J.M.; Talaska G.; Tang M.S.; Thakur N.; van Tongeren M.; Vineis P.:
IARC Monographs Vol 128 group. Carcinogenicity of acrolein, crotonaldehyde, and arecoline.
Lancet Oncol. 2021, 22, 19-20. doi: 10.1016/S1470-2045(20)30727-0 - Jágerová, D.; Šmahel, M.; Poryvai, A.; Macháček, J.; Novotná, V.; Kohout, M.:
Photosensitive bent-core liquid crystals with laterally substituted azobenzene unit. Crystals, 2021, 11, 1265.
https://doi.org/10.3390/cryst11101265 - Kohout, M.;J. Mol. Liq. 2021, 339, 116744 DOI:10.1016/j.molliq.2021.116744
- Karongo, R.; Ge, M.; Horak, J.; Gross, H.; Kohout, M.; Lindner, W.; Lämmerhofer, M.:
Rapid enantioselective amino acid analysis by ultra-high performance liquid chromatography-mass spectrometry combining 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate derivatization with core-shell quinine carbamate anion exchanger separation. J. Chromatogr. Open, 2021, 1, 100004.
https://doi.org/10.1016/j.jcoa.2021.100004 - Herciková, J.; Spálovská, D.; Frühauf, P.; Izák, P.; Lindner, W.; Kohout, M.:
Design and synthesis of naphthalene-based chiral strong cations exchangers and their application for chiral separation of basic drugs. J. Sep. Sci., 2021, 44, 3348-3356. DOI: 10.1002/jssc.202100127
2020
- Poryvai A., Bubnov A., Kohout M.:
Chiral Photoresponsive Liquid Crystalline Materials Derived from Cyanoazobenzene Central Core: Effect of UV Light Illumination on Mesomorphic Behavior
Crystals 2020, 10, 1161. https://doi.org/10.3390/cryst10121161 - Skopalová H., Špaček P., Kozmík V., Svoboda J., Novotná V., Pociecha D., Kohout M.:
The Role of Substitution in the Apex Position of the Bent-Core on Mesomorphic Properties of New Series of Liquid Crystalline Materials
Crystals, 2020, 10, 735. https://doi.org/10.3390/cryst10090735 - Guselnikova O., Postnikov P., Kolská Z., Záruba K., Kohout M., Elashnikov R., Švorčík V., Lyutakov O.:
Homochiral metal-organic frameworks functionalized SERS substrate for atto-molar enantio-selective detection
Appl. Mater. Today 2020, 20, 100666. https://doi.org/10.1016/j.apmt.2020.100666 - Kolderová N., Jurásek B., Kuchař M., Lindner W., Kohout M.:
Gradient supercritical fluid chromatography coupled to mass spectrometry with a gradient flow of make-up solvent for enantioseparation of cathinones
J. Chromatogr. A 2020, 1625, 461286. https://doi.org/10.1016/j.chroma.2020.461286 - Šmahel M., Poryvai A., Xiang Y., Pociecha D., Troha T., Novotná V., Svoboda J., Kohout M.:
Photosensitive bent-core nematic liquid crystals with various linking units in the side arms: Structure-properties relationships
J. Mol. Liq. 2020, 306, 112743. https://doi.org/10.1016/j.molliq.2020.112743 - Cigl M., Jurok R., Hampl F., Svoboda J., Podoliak N., Novotná V.:
Lateral substituted phenyl biphenylcarboxylates ‒ non-chiral analogues of ferroelectric liquid crystals
Liq. Cryst. 2020, 47, 768-776. https://doi.org/10.1080/02678292.2019.1679903 - Gaálová J., Yalcinkaya F., Cuřínová P., Kohout M., Yalcinkaya B., Koštejn M., Jirsák J., Stibor I., Bara J. E., Van der Bruggen B., Izák P.:
Separation of racemic compound by nanofibrous composite membranes with chiral selector
J. Membr. Sci. 2020, 596, 117728. https://doi.org/10.1016/j.memsci.2019.117728 - Otmar M., Gaálová J., Žitka J., Brožová L., Cuřínová P., Kohout M., Hovorka Š., Bara J. E., Van der Bruggen B., Jirsák J., Izák P.:
Preparation of PSEBS membranes bearing (S)-(-)-methylbenzylamine as chiral selector
Eur. Polym. J. 2020, 122, 109381. https://doi.org/10.1016/j.eurpolymj.2019.109381 - Oeser, P.; Koudelka, J.; Dvořáková, H.; Tobrman, T.:
Formation of trisubstituted buta-1,3-dienes and α,β-unsaturated ketones via the reaction of functionalized vinyl phosphates and vinyl phosphordiamidates with organometallic reagents.
RSC Adv. 2020, 10, 35109-35120. DOI: 10.1039/D0RA07472A - Čubiňák, M.; Tobrman, T.:
Room-Temperature Negishi Reaction of Trisubstituted Vinyl Phosphates for the Synthesis of Tetrasubstituted Alkenes.
J. Org. Chem. 2020, 85, 10728-10739. DOI: 10.1021/acs.joc.0c01254 - Polák, P.; Čejka, J.; Tobrman, T.:
Formal Transition-Metal-Catalyzed Phosphole C–H Activation for the Synthesis of Pentasubstituted Phospholes.
Org. Lett. 2020, 22, 2187─2190 DOI: 10.1021/acs.orglett.0c00359 - Tobrman, T; Krupička, M.; Polák, P.; Dvořáková, H.; Čubiňák, M.; Babor, M.; Dvořák, D.:
Diastereoselective Cyclopropanation through Michael Addition‐Initiated Ring Closure between α,α‐Dibromoketones and α,β‐Unsaturated Fischer Carbene Complexes.
Eur. J. Org. Chem. 2020, 429─436 DOI: 10.1002/ejoc.201901503 - Guricová, M.; Tobrman, T.; Pižl, M.; Žižková, S.; Hoskovcová, I.; Dvořák, D.:
Synthesis, characterisation and electrochemical properties of Cr(0) aminocarbene complexes containing condensed heteroaromatic moiety.
Organomet. Chem. 2020, 905, 121023 DOI: 10.1016/j.jorganchem.2019.121023
- Kortus, D.; Kundrat, O.; Tlusty, M.; Cejka, J.; Dvorakova, H.; Lhotak, P.:
Inherent chirality through a simple dialkylation of 2,14-dithiacalix[4]arene
New J. Chem. 2020, 44, 14496-14504 DOI:10.1039/d0nj03468a - Tlusty, M.; Spalovska, D.; Kohout, M.; Eigner, V.; Lhotak, P.:
Ketone transformation as a pathway to inherently chiral rigidified calix[4]arenes
Chem. Commun. 2020, 56, 12773-12776 DOI:10.1039/d0cc05352j - Kolarikova, V.; Rybackova, M.; Svoboda, M.; Kvicala, J.:
Ring-closing metathesis of prochiral oxaenediynes to racemic 4-alkenyl-2-alkynyl-3,6-dihydro-2H-pyrans
Beilstein J. Org. Chem. 2020, 16, 2757–2768 DOI:10.3762/bjoc.16.226 - Simunek, O.; Rybackova, M.; Svoboda, M.; Kvicala, J.:
Synthesis, catalytic activity and medium fluorous recycle of fluorous analogues of PEPPSI catalysts
J. Fluorine Chem. 2020, 236, 109588 DOI:10.1016/j.jfluchem.2020.109588 - Graml, A.; Neveselý, T.; Kutta, R.-J.; Cibulka, R.; König, B.:
Deazaflavin reductive photocatalysis involves excited semiquinone radicals
Nature Comm. 2020, 11, 3174. DOI: 10.1038/s41467-020-16909-y - Regioselective SNAr reaction of the phenoxathiin-based thiacalixarene as a route to a novel macrocyclic skeleton
Chem. Commun. 2020, 56, 78-81 DOI:10.1039/c9cc08335a - Tlusty, M.; Dvorakova, H.; Cejka, J.; Kohout, M.; Lhotak, P.:
Regioselective formation of the quinazoline moiety on the upper rim of calix[4]arene as a route to inherently chiral systems
New J. Chem. 2020, 44, 6490-6500 DOI:10.1039/d0nj01035a - Máková, V.; Holubová, B.; Tetour, D.; Brus, J.; Řezanka, M.; Rysová, M.; Hodačová, J.:
(1S,2S)‐Cyclohexane‐1,2‐diamine‐based Organosilane Fibres as a Powerful Tool Against Pathogenic Bacteria
Polymers 2020, 12, 206 DOI:10.3390/polym12010206 - Cibulka, R.:
Strong chemical reducing agents produced by light
Nature 2020, 580, 31-32 DOI: 10.1038/d41586-020-00872-1 - Tolba, A. H.; Vávra, F.; Chudoba, J.; Cibulka, R.:
Tuning flavin-based photocatalytic systems for application in the mild chemoselective aerobic oxidation of benzylic substrates
Eur. J. Org. Chem. 2020, 1579–1585 DOI:10.1002/ejoc.201901628 Special issue Photochemical synthesis
- Schlosser J., Cibulka R., Groß P., Ihmels H., Mohrschladt C. J.:
Visible-light induced di-π-methane rearrangement of dibenzobarrelene derivatives
ChemPhotoChem 2020, 4, 132-137 DOI: 10.1002/cptc.201900221 - Zelenka, J.; Cibulka, R.; Roithová, J.:
Flavinium catalyzed photooxidation: Detection and characterization of elusive peroxyflavinium intermediates
Angew. Chem. Int. Ed. 2020, 58, 15412-15420 DOI: 10.1002/anie.201906293
COVER:
- Mráz J., Hanzlíková I., Dušková Š., Tvrdíková M., Linhart I.:
N-(2-Hydroxyethyl)-l-valyl-l-leucine: a novel urinary biomarker of ethylene oxide exposure in humans.
Toxicol Lett. 2020, 15, 18-22. doi: 10.1016/j.toxlet.2020.03.004
2019
- Slavik, P.; Krupicka, M.; Eigner, V.; Vrzal, L.; Dvorakova, H.; Lhotak, P.:
Rearrangement of meta-Bridged Calix[4]arenes Promoted by Internal Strain
J. Org. Chem. 2019, 84, 4229-4235, DOI:10.1021/acs.joc.9b00107 - Kortus, D.; Miksatko, J.; Kundrat, O.; Babor, M.; Eigner, V.; Dvorakova, H.; Lhotak, P.:
Chemistry of 2,14-Dithiacalix[4]arene: Alkylation and Conformational Behavior of Peralkylated Products
J. Org. Chem. 2019, 84, 11572-11580, DOI:10.1021/acs.joc.9b01493 - Tlusty, M.; Eigner, V.; Babor, M.; Kohout, M.; Lhotak, P.:
Synthesis of Upper Rim-double-bridged Calix[4]arenes bearing Seven Membered Rings and Related Compounds
RSC Advances 2019, 9, 22017-22030 DOI:10.1039/c9ra05075b - Pokluda, A.; Kohout, M.; Chudoba, J.; Krupička, M.; Cibulka, R.:
Nitrosobenzene – Reagent for the Mitsunobu Esterification Reaction
ACS Omega 2019, 4, 5012-5018. DOI: 10.1021/acsomega.8b03551 - Valenta, L.; Kovaricek, P.; Vales, V.; Bastl, Z.; Drogowska, K. A.; Verhagen, T. A.; Cibulka, R.; Kalbac, M.:
Spatially resolved covalent functionalization patterns on graphene
Angew. Chem. Int. Ed. 2019, 58, 1324-1328 DOI: 10.1002/anie.201810119 - Zelenka, J.; Svobodová, E.; Tarábek, J.; Hoskovcová, I.; Boguschová, V.; Bailly, S.; Sikorski, M.; Roithová, J.; Cibulka, R.:
Combining flavin photocatalysis and organocatalysis: metal-free aerobic oxidation of unactivated benzylic substrates
Org. Lett. 2019, 21, 114-119 DOI: 10.1021/acs.orglett.8b03547
Highly cited paper (WOS) - Jing, H.; Xu, M.; Xiang, Y.; Wang, E.; Liu, D.; Poryvai, A.; Kohout, M.; Éber, N.; Buka, A.:
Light tunable gratings based on flexoelectric effect in photoresponsive bent-core nematics.
Adv. Opt. Mater. 2019, 1801790 DOI: 10.1002/adom.201801790 - Pokluda, A.; Kohout, M.; Chudoba, J.; Krupička, M.; Cibulka, R.:
Nitrosobenzene: Reagent for the Mitsunobu esterification reaction.
ACS Omega 2019, 4, 5012-5018 DOI: 10.1021/acsomega.8b03551 - Spálovská, D.; Maříková, T.; Kohout, M.; Králík, F.; Kuchař, M.; Setnička, V.:
Methylone and pentylone: Structural analysis of new psychoactive substances.
Forensic Toxicol. 2019, 37, 366-377 DOI: 10.1007/s11419-019-00468-z - Poryvai, A.; Bubnov, A.; Pociecha, D.; Svoboda, J.; Kohout, M.:
The effect of the length of terminal n-alkyl carboxylate chain of self-assembling and photosensitive properties of chiral lactic acid derivatives.
J. Mol. Liq. 2019, 275, 829-838 DOI: 10.1016/j.molliq.2018.11.058 - Geibel, C.; Dittrich, K.; Woiwode, U.; Kohout, M.; Zhang, T.; Lindner, W.; Lämmerhofer, M.:
Evaluation of superficially porous particle based zwitterionic chiral ion exchangers against fully porous particle benchmarks for enantioselective ultra-high performance liquid chromatography.
J. Chromatogr. A 2019, 1603, 130-140 DOI: 10.1016/j.chroma.2019.06.026 - Poryvai, A.; Vojtylová-Jurkovičová, T.; Šmahel, M.; Kolderová, N.; Tomášková, P.; Sýkora, D.; Kohout, M.:
Determination of optical purity of lactic acid-based chiral liquid crystals and corresponding building blocks by chiral high-performance liquid chromatography and supercritical fluid chromatography.
Molecules 2019, 24, 1099 DOI: 10.3390/molecules24061099 - Tlustý, M.; Eigner, V.; Babor, M.; Kohout, M.; Lhoták, P.:
Synthesis of upper rim-double-bridged calix[4]arenes bearing seven membered rings and related compounds.
RSC Adv. 2019, 9, 22017-22030. DOI: 10.1039/C9RA05075B - Mráz J., Hanzlíková I., Linhart I., Dušková Š., Dabrowská L., Hejl K.:
N-(2-Hydroxyethyl)-L-valyl-L-leucine in rat urine as a hydrolytic cleavage product of ethylene oxide adduct with globin.
Arch Toxicol. 2019, 93, 603-613. doi: 10.1007/s00204-019-02388-8 - Linhart, I.:
Chemická legislativa pohledem laboratorního chemika.
Chemické listy 2019, 113, 140-148.
2018
- Jurásek, B.; Králík, F.; Rimpelová S.; Čejka, J.; Setnička, V.; Ruml, T.; Kuchař, M.; Kohout, M.:
Synthesis, Absolute Configuration and in vitro Cytotoxicity of Deschloroketamine Enantiomers: Rediscovered and Abused Dissociative Anaesthetic
New. J. Chem. 2018, 42, 19360-19368 DOI: 10.1039/c8nj03107j
COVER:
- Landovsky, T.; Dvorakova, H.; Eigner, V.; Babor, M.; Krupicka, M.; Kohout, M.; Lhotak, P.:
Chemoselective Oxidation of Phenoxathiin-based Thiacalix[4]arene and the Stereoselective Alkylation of Products
New J. Chem. 2018, 42, 20074-20086 DOI: 10.1039/C8NJ04690E - Mojr, V.; Pitrova, G.; Strakova, K.; Prukala, D.; Brazevic, S.; Svobodová, E.; Hoskovcová, I.; Burdziński, G.; Slanina, T.; Sikorski, M.; Cibulka, R.:
Flavin Photocatalysts for visible Light [2+2] Cycloadditions: Structure, Reactivity and Reaction Mechanism
ChemCatChem 2018, 10, 849-858 DOI: 10.1002/cctc.201701490 - Marz, M.; Kohout, M.; Nevesely, T.; Chudoba, J.; Prukala, D.; Nizinski, S.; Sikorski, M.; Burdzinski, G.; Cibulka, R.:
Azodicarboxylate-free esterification with triphenylphosphine mediated by flavin and visible light: method development and stereoselectivity control
Org. Biomol. Chem. 2018, 16, 6809-6817 DOI:10.1039/C8OB01822G - Mundil, R.; Sokolohorskyj, A.; Hosek, J.; Cvacka, J.; Cisarova, I.; Kvicala, J.; Merna, J.:
Nickel and palladium complexes with fluorinated alkyl substituted α-diimine ligands for living/controlled olefin polymerization
Polym. Chem. 2018, 9, 1234-1248 DOI: 10.1039/C8PY00201K - Hosek, J.; Simunek, O.; Lipovska, P.; Kolarikova, V.; Kucnirova, K.; Edr, A.; Stepankova, N.; Rybackova, M.; Cvacka, J.; Kvicala, J.:
Medium Fluorous Separation Using Hydrofluoroether and Weakly Polar Solvents for Environmentally Friendly Recycling of Catalysts
ACS Sustainable Chem. Eng. 2018, 6, 7026-7034 DOI:10.1021/acssuschemeng.8b00865 - Kucnirova, K.; Simunek, O.; Rybackova, M.; Kvicala, J.:
Structural Assignment of Fluorocyclobutenes by 19F NMR Spectroscopy – Comparison of Calculated 19F NMR Shielding Constants with Experimental 19F NMR Shifts
Eur. J. Org. Chem. 2018, 3867-3874 DOI:10.1002/ejoc.201800482 - Slavik, P.; Eigner, V.; Lhotak, P.:
meta-Bridged calix[4]arenes with the methylene moiety possessing in/out stereochemistry of substituents
New J. Chem. 2018 asap DOI:10.1039/c8nj02577k - Landovsky, T.; Tichotova, M.; Vrzal, L.; Budka, J.; Eigner, V.; Dvorakova, H.; Lhotak, P.:
Structure elucidation of phenoxathiin-based thiacalix[4]arene conformations using NOE and RDC data
Tetrahedron 2018, 74, 902-907 DOI:10.1016/j.tet.2018.01.020 - Slavik, P.; Dvorakova, H.; Krupicka, M.; Lhotak, P.:
Unexpected cleavage of upper rim-bridged calix[4]arenes leading to linear oligophenolic derivatives
Org. Biomol. Chem. 2018, 59, 838-843 DOI:10.1039/C7OB03101G - Slavik, P.; Lhotak, P.:
Unusual reactivity of upper-rim bridged calix[4]arenes - Friedel-Crafts alkylation via cleavage of the macrocyclic skeleton
Tetrahedron 2018, 59, 1757-1759 DOI:10.1016/j.tetlet.2018.03.072
- Schmitt, K.; Woiwode, U.; Kohout, M.; Zhang, T.; Lindner, W.; Lämmerhofer, M.:
Comparison of small size fully porous particles and superficially porous particles of chiral anion-exchange type stationary phases in ultra-high performance liquid chromatography: effect of particle and pore size on chromatographic efficiency and kinetic performance
J. Chromatogr. A 2018, 1569, 149-159 DOI:10.1016/j.chroma.2018.07.056
- Spálovská, D.; Kralik, F.; Kohout, M.; Jurasek, B.; Habartová, L.; Kuchar, M.; Setnicka, V.:
Structure determination of butylone as a new psychoactive substance using chiroptical and vibrational spectroscopies
Chirality 2018, 30, 548-559 DOI:10.1002/chir.22825 - Wolrab, D.; Kohout, M.:
Multimodální stacionární fáze pro kapalinovou chromatografii, způsob jejich přípravy a jejich použití.
Národní patent č. 307339, číslo přihlášky 2017-193, datum udělení 2.5.2018, datum zveřejnění 13.6.2018. - Kohout, M.; Wernisch, S.; Tuma, J.; Hettegger, H.; Picha, J.; Lindner W.:
Effect of different immobilization strategies on chiral recognition properties of Cinchona-based anion exchangers
J. Sep. Sci. 2018, 41, 1355-1364 DOI:10.1002/jssc.201701213 - Bajtai, A.; Fekete, B.; Palkó, M.; Fülöp, F.; Lindner, W.; Kohout, M.; Ilisz, I.; Péter, A.:
Comparative study on the liquid chromatographic enantioseparation of cyclic β-amino acids and the related cyclic β-aminohydroxamic acids on Cinchona alkaloid-based zwitterionic chiral stationary phases
J. Sep. Sci. 2018, 41, 1216-1223 DOI:10.1002/jssc.201701190 - Sardella, R.; Macchiarulo, A.; Urbinati, F.; Ianni, F.; Carotti, A.; Kohout, M.; Lindner, W.; Péter, A.; Ilisz, I.:
Exploring the enantiorecognition mechanism of Cinchona alkaloid-based zwitterionic chiral stationary phases and the basic trans-paroxetine enantiomers
J. Sep. Sci. 2018, 41, 1199-1207 DOI:10.1002/jssc.201701068 - Tuma, J.; Kohout, M.:
Silica gel-immobilized multidisciplinary materials applicable in stereoselective organocatalysis and HPLC separation
RSC Adv. 2018, 8, 1174-1181 DOI:10.1039/c7ra12658a - Kozmik, V.; Rodinová, E.; Prausová, T.; Svoboda, J.; Novotná, V.; Pociecha, D.:
Mesogens with central naphthalene core substituted at various positions
Liq. Cryst. 2018, 45, 744-756 DOI: 10.1080/02678292.2017.1380238 - Bajzíková, K.; Vesely, J.; Kozmik, V.; Svoboda, J.; Novotná, V.; Pociecha, D.:
Thiophene central core for the design of bent-shaped liquid crystals
J. Mol. Liq. 2018, 267, 496-503 DOI:10.1016/j.molliq.2018.02.009 - Horcic, M.; Svoboda, J.; Novotná, V.; Pociecha, D.; Gorecka, E.:
Bent-core dimers with top-to-bottom linkage between central units
RSC Adv. 2018, 8, 22974-22985 DOI: 10.1039/c8ra04108c - Mráz J, Hanzlíková I, Dušková Š, Tvrdíková M, Chrástecká H, Vajtrová R, Linhart I.:
Determination of N-(2-hydroxyethyl)valine in globin of ethylene oxide-exposed workers using total acidic hydrolysis and HPLC-ESI-MS2
Toxicol Lett. 2018, 298, 76-80. doi: 10.1016/j.toxlet.2018.06.1212
2017
- Maixner, J.; Jurásek, B.; Kohout, M.; Kuchar, M.; Kacer, P.:
X-ray powder diffraction data for (S)-Deschloroketamine hydrochloride, C13H18ClNO.
Powder Diffr. 2017, 32, 193-195. DOI:10.1017/S0885715617000586 - Bajzíková, K.; Svoboda, J.; Novotná, V.; Pociecha, D.; Gorecka E.:
Bent-core mesogens with an aromatic unit at the terminal position
New J. Chem. 2017, 41, 4672-4679 DOI: 10.1039/C6NJ03908A - Žurek, J.; Svobodová, E.; Šturala, J.; Dvořáková, H.; Svoboda, J.; Cibulka, R.:
Chiral ethylene-bridged flavinium salts: The stereoselectivity of flavin-10a-hydroperoxide formation and the effect of substitution on the photochemical properties
Tetrahedron Asymmetry 2017, asap DOI:10.1016/j.tetasy.2017.10.029 - Muchová; E.; Bezek, M.; Suchan, J.; Cibulka, R.; Slavíček, P.:
Molecular Dynamics and Metadynamics Simulations of [2+2] Photocycloaddition
Int. J. Quantum Chem. 2017, asap DOI:10.1002/qua.25534 - Dobrovolny, K.; Ulbrich, P.; Svecova, M.; Rimpelova, S.; Malincik, J.; Kohout, M.; Svoboda, J.; Bartunek, V.:
Copper nanoparticles in glycerol-polyvinyl alcohol matrix: In situ preparation, stabilization and antimicrobial activity
Journal of Alloys and Compounds 2017, 697, 147-155 DOI:10.1016/j.jallcom.2016.12.144 - Kolderova, N.; Nevesely, T.; Sturala, J.; Kuchar, M.; Holakovsky, R.; Kohout, M.:
Enantioseparation of chiral sulfoxides on amylose-based columns: comparison of normal phase liquid chromatography and supercritical fluid chromatography
Chromatographia 2017, 80, 547-557 DOI: 10.1007/s10337-016-3234-6 - Kohout, M.; Alaasar, M.; Poryvai, A.; Novotna, V.; Poppe, S.; Tschierske, C.; Svoboda, J.:
Photosensitive bent-core liquid crystals based on methyl substituted 3-hydroxybenzoic acid
RSC Advances 2017, 7, 35805-35813 DOI:10.1039/C7RA05632J - Wolrab, D.; Fruehauf, P.; Gerner, C.; Kohout, M.; Lindner, W.:
Consequences of transition from liquid chromatography to supercritical fluid chromatography on the overall performance of a chiral zwitterionic ion-exchanger
Journal of Chromatography A 2017, 1517, 165-175 DOI:10.1016/j.chroma.2017.08.022 - Pallova, L.; Kozmik, V.; Kohout, M.; Svoboda, J.; Novotna, V.; Pociecha, D.:
Bent-core liquid crystals with a 2-substituted 3-hydroxybenzoic acid central core
Liquid Crystals 2017, 44, 1306-1315 DOI: 10.1080/02678292.2016.1276981 - Zidkova, M.; Linhart, I.; Balikova, M.; Himl, M.; Dvorackova, V.; Lhotkova, E.; Palenicek, T.:
Identification of three new phase II metabolites of a designer drug methylone formed in rats by N-demethylation followed by conjugation with dicarboxylic acids
Xenobiotica 2017, asap DOI: 10.1080/00498254.2017.1349964 - Linhart, I.; Hanzlikova, I.; Mraz, J.; Duskova, S.:
S-(3-Aminobenzanthron-2-yl)cysteine in the globin of rats as a novel type of adduct and possible biomarker of exposure to 3-nitrobenzanthrone, a potent environmental carcinogen
Archives of Toxicology 2017, asap DOI:10.1007/s00204-017-1943-8 - Polak, P.; Dvorak, D.; Tobrman, T.:
Cyanogen: A Versatile Reagent for Diversity-Oriented Synthesis
Synthesis 2017, 49, 1757-1766 DOI:10.1055/s-0036-1588410 - Polak, P.; Tobrman, T.:
The synthesis of polysubstituted indoles from 3-bromo-2-indolyl phosphates
Org. Biomol. Chem. 2017, 15, 6233-6241 DOI:10.1039/C7OB01127J - Polak, P.; Tobrman, T.:
Dearomatization Strategy for the Synthesis of Arylated 2H-Pyrroles and 2,3,5-Trisubstituted 1H-Pyrroles
Org. Lett. 2017, 19, 460-4611 DOI: 10.1021/acs.orglett.7b02219 - Simunkova, N.; Tobrman, T.; Eigner, V.; Dvorak, D.:
A Study on the Intramolecular Mitsunobu Reaction of N6-(ω-hydroxyalkyl)adenines
J. Heterocyclic Chem 2017, asap DOI: 10.1002/jhet.2982 - Bregier, F.; Hudecek, O.; Chaux, F.; Penouilh, M.; Chambron, J.; Lhotak, P.; Aubert, E.; Espinosa, E.:
Generation of Cryptophanes in Water by Disulfide Bridge Formation
Eur. J. Org. Chem. 2017, 3795-3811 DOI:10.1002/ejoc.201700537 - Tlusty, M.; Slavik, P.; Kohout, M.; Eigner, V.; Lhotak, P.:
Inherently Chiral Upper-Rim-Bridged Calix[4]arenes Possessing a Seven Membered Ring
Org. Lett. 2017, 19, 2933-2936 DOI:10.1021/acs.orglett.7b01170 - Jirasek, M.; Strakova, K.; Nevesely, T.; Svobodova, E.; Rottnerova, Z.; Cibulka, R.:
Flavin-mediated visible light [2+2] photocycloaddition of nitrogen and sulfur-containing dienes
Eur. J. Org. Chem. 2017, 2139-2146 DOI:10.1002/ejoc.201601377 - Slavik, P.; Eigner, V.; Bohm, S.; Lhotak, P.:
Mercuration of Calix[4]arene Immobilized in the 1,2- and 1,3-Alternate Conformations
Tetrahedron Letters 2017, 58, 1846-1850 DOI:10.1016/j.tetlet.2017.03.085 - Miksatko, J.; Eigner, V.; Kohout, M.; Lhotak, P.:
Regio-/stereoselective Formation of Monosulfoxides from Thiacalix[4]arenes in All Possible Conformations
Tetrahedron Letters 2017, 58, 1687-1691 DOI:10.1016/j.tetlet.2017.03.043 - März, M.; Chudoba, J.; Kohout, M.; Cibulka, R.:
Photocatalytic Esterification under Mitsunobu Reaction Conditions Mediated by Flavin and Visible Light
Org. Biomol. Chem. 2017, 15, 1970-1975 DOI:10.1039/C6OB02770A - Spackova, J.; Svobodova, E.; Hartman, T.; Stibor, I.; Kopecka, J.; Cibulkova, J.; Chudoba, J.; Cibulka, R.:
Visible light light [2+2] Photocycloaddition mediated by flavin derivative immobilized on mesoporous silica
ChemCatChem. 2017, 9, 1177-1181 DOI:10.1002/cctc.201601654 - Horčic, M.; Kohout, M.; Svoboda, J.; Novotna, V.; Pociecha, D.; Gorecka, E.:
Core-to-core Dimers Forming Switchable Mesophase
Chem. Commun. 2017, 53, 2721-2724 DOI:10.1039/C6CC09983A - Tlusty, M.; Slavik, P.; Dvorakova, H.; Eigner, V.; Lhotak, P.:
Synthesis and Study of Calix[4]arenes bearing Azo Moieties at the meta Position
Tetrahedron 2017, 73, 1230-1237 DOI:10.1016/j.tet.2017.01.025 - Rezankova, M.; Budka, J.; Miksatko, J.; Eigner, V.; Cisarova, I.; Curinova, P.; Lhotak, P.:
Anion Receptors based on Intramolecularly Bridged Calix[4]arenes bearing Ureido Functions
Tetrahedron 2017, 73, 742–749 DOI:10.1016/j.tet.2016.12.054
2016
- Bajzikova, K.; Kohout, M.; Tarabek, J.; Svoboda, J.; Novotna, V.; Vejpravova, J.; Pociecha, D.; Gorecka, E.:
All-organic liquid crystalline radicals with a spin unit in the outer position of a bent-core system
J. Mater. Chem. C 2016, 4, 11540-11547 DOI:10.1039/C6TC04346A - Zidkova, M.; Linhart, I.; Balikova, M.; Himl, M.; Vana, L.; Vetyska, M.; Palenicek, T.; Lhotkova, E.; Dusek, M.:
Study on metamolism of 5,6-methylenedioxy-2-aminoindane (MDAI) in rats: identification of urinary metabolites
Xenobiotica 2016, asap DOI:10.1080/00498254.2016.1199919 - Vanova, H.; Tobrman, T.; Hoskovcova, I.; Dvorak, D.:
Modular synthesis of Fischer biscarbene complexes of chromium
Organometallics 2016, 35, 2999-3006 DOI:10.1021/acs.organomet.6b00527 - Mraz, J.; Hanzlikova, I.; Duskova, S.; Dabrowska, L.; Chrastecka, H.; Vajtrova, R.; Linhart, I.:
Biological fate of styrene oxide adducts with globin: Elimination of cleavage products in the rat urine
Toxicology Letters 2016, 261, 26-31 DOI:10.1016/j.toxlet.2016.08.022 - Lipovska, P.; Rathouska, L.; Simunek, O.; Hosek, J.; Kolarikova, V.; Rybackova, M.; Cvacka, J.; Svoboda, M.; Kvicala, J.:
Synthesis and catalytic activity of ruthenium complexes modified with chiral racemic per- and polyfluorooxaalkanoates
J. Fluorine Chem. 2016, 191, 14-22 DOI:10.1016/j.jfluchem.2016.09.005 - Kotek, V.; Polák, P.; Dvořáková, H.; Tobrman, T.:
Aluminium Chloride Promoted Cross-Coupling of Trisubstituted Enol Phosphates with Organozinc Reagents En Route to the Stereoselective Synthesis of Tamoxifen and Its Analogues
Eur. J. Org. Chem. 2016, 5037-5044 DOI:10.1002/ejoc.201600959 - Tůma, J.; Kohout, M.; Svoboda, J.; Novotná, V.; Pociecha, D.:
Bent-core liquid crystals based on 6-substituted 3-hydroxybenzoic acid: the role of substitution and linkage group orientation on mesomorphic properties
Liq. Cryst. 2016, 43, 1889-1900 DOI:10.1080/02678292.2016.1230789 - Slavik, P.; Eigner, V.; Lhotak, P.:
A General Method for Obtaining Calixarene Derivatives in the 1,2-Alternate Conformation
Tetrahedron 2016, 72, 6348-6355 DOI:10.1016/j.tet.2016.08.028 - Liska, A.; Lhotak, P.; Ludvik, J.:
Electrochemical Reduction and Intramolecular Electron Communication of Nitro Substituted Thiacalix[4]arene
Electroanalysis 2016, 28, 2861-2865 DOI:10.1002/elan.201600342 - Klejch, T.; Slavicek, J.; Hudecek, O.; Eigner, V.; Gutierrez, N. A.; Curinova, P.; Lhotak, P.:
Calix[4]arene Containing a Ureido Functionality on the Lower Rim as Highly Efficient Receptors for Anion Recognition
New J. Chem. 2016, 40, 7935-7942 DOI:10.1039/c6nj01271j - Kohout, M.; Vandenbussche, J.; Roller, A.; Tůma, J.; Boqaerts, J.; Bultinck, P.; Herrebout, W.; Lindner, W.:
Absolute configuration of the antimalarial erythro-mefloquine - vibrational dichroism and X-ray diffraction studies of mefloquine and its thiourea derivative
RSC Advances 2016, 6, 81461-81465 DOI:10.1039/C6RA19367F - Polak, P.; Vanova, H.; Dvorak, D.; Tobrman, T.:
Recent Progress in Transition Metal-Catalyzed Stereoselective Synthesis of Acyclic All-Carbon Tetrasubstituted Alkenes
Tetrahedron Lett. 2016, 57, 3684-3693 DOI:10.1016/j.tetlet.2016.07.030 - Navratil, R.; Tarabek, J.; Linhart, I.; Martinů, T.:
Radical and Nitrenoid Reactivity of 3-Halo-3-phenyldiazirines
Org. Lett. 2016, 18, 3734–3737 DOI:10.1021/acs.orglett.6b01753 - Miksatko, J.; Eigner, V.; Dvorakova, H.; Lhotak, P.:
Selective Oxidation of Thiacalix[4]arene (cone) to all Corresponding Sulfoxides
Tetrahedron Lett. 2016, 57, 3781–3784 DOI:10.1016/j.tetlet.2016.07.022 - Hartman, T.; Cibulka, R.:
Photocatalytic systems with flavinium salts: From photolyase models to synthetic tool for cyclobutane ring opening
Org. Lett. 2016, 18, 3710-3713 DOI:10.1021/acs.orglett.6b01743 - Bím, D.; Svobodová, E.; Eigner, V.; Rulíšek, L.; Hodačová, J.:
Copper(II) and Zinc(II) Complexes of Conformationally Constrained Polyazamacrocycles as Efficient Catalysts for RNA Model Substrate Cleavage in Aqueous Solution at Physiological pH
Chem. Eur. J. 2016, 22, 10426-10437 DOI:10.1002/chem.201601175
- Kohout, M.; Bubnov, A.; Šťurala, J.; Novotná, V.; Svoboda, J.:
Effect on alkyl chain length in the terminal ester group on mesomorphic properties of new chiral lactic acid derivatives
Liq. Cryst. 2016, 43, 1472-1485 DOI:10.1080/02678292.2016.1185170 - Grecsó, N.; Kohout, M.; Carotti, A.; Sardella, R.; Natalini, B.; Fülöp, F.; Lindner, W.; Péter, A.; Ilisz, I.:
Mechanistic considerations of enantiorecognition on novel Cinchona alkaloid-based zwitterionic chiral stationary phases from the aspect of the separation of trans-paroxetine enantiomers as model compounds
J. Pharm. Biomed. Anal. 2016, 124, 164-173 DOI:10.1016/j.jpba.2016.02.043 - Wolrab, D.; Frühauf, P.; Moulisova, A.; Kuchar, M.; Gerner, C.; Lindner, W.; Kohout, M.:
Chiral separation of new designer drugs (Cathinones) on chiral ion-exchange type stationary phases
J. Pharm. Biomed. Anal. 2016, 120, 306-315 DOI:10.1016/j.jpba.2015.12.023
- Slavik, P.; Eigner, V.; Lhotak, P.:
Shaping of Calix[4]arenes via Double Bridging of the Upper Rim
Cryst. Eng. Comm. 2016, 18, 4964-4970 DOI:10.1039/C6CE00314A
- Neveselý, T.; Svobodová, E.; Chudoba, J.; Sikorski, M.; Cibulka, R.:
Efficient metal-free aerobic photooxidation of sulfides to sulfoxides mediated by a vitamin B2 derivative and visible light
Adv. Synth. Catal. 2016, 358, 1654-1663 DOI:10.1002/adsc.201501123 - Mraz, J.; Hanzlikova, I.; Moulisova, A.; Duskova, S.; Hejl, K.; Bednarova, A.; Dabrowska, L.; Linhart, I.:
Hydrolytic cleavage products of globin adducts in urine as possible biomarkers of cumulative dose: Proof of concept using styrene oxide as a model adduct-forming compound
Chem. Res. Toxicol. 2016, 29, 676-686 DOI:10.1021/acs.chemrestox.5b00518 - Dolenský, B.; Kvíčala, J.; Paleta, O.:
Methyl fluoroalkanoate as methyl-transferring reagent. Unexpected participation of BAl2 (SN2) mechanism in the reaction of methyl 2,3,3,3-tetrafluoro-2-methoxypropanoate with amines.
J. Fluorine Chem. 2016, 185, 31-35. DOI:10.1016/j.jfluchem.2016.02.012 - Kundrat, O.; Slavik, P.; Miksatko, J.:
Introducing an Amine Group to Calix[5]arene: Comparison of Several Methods
Supramol. Chem. 2016, 28, 450-454, DOI:10.1080/10610278.2015.1119275 - Tůma, J.; Kohout, M.; Svoboda, J.; Novotná, V.; Pociecha, D.:
Bent-shaped liquid crystals based on 4-substituted 3-hydroxybenzoic acid central core - Part II
Liq. Cryst. 2016, 43, 547-563, DOI: 10.1080/02678292.2015.1125535 - Kotek, V.; Polák, P.; Tobrman, T.:
Efficient and Simple Preparation of Functionalized 1,1-Dibromoenol Phosphates
Monatshefte fuer Chemie 2016, 147, 405-412, DOI: 10.1007/s00706-015-1613-6 - Linhart, I.; Himl, M.; Zidkova, M.; Balikova, M.; Lhotakova, E.; Palenicek, T.:
Metabolic Profile of Mephedrone: Identification of Normephedrone Conjugates with Dicarboxylic Acids as a New Type of Xenobiotic Phase II Metabolites
Toxicology Letters 2016, 240, 114-121, DOI: 10.1016/j.toxlet.2015.10.025 - Botha, F.; Eigner, V.; Dvorakova, H.; Lhotak, P.:
Arylation of Thiacalix[4]arenes using Organomercurial Intermediates
New J. Chem. 2016, 40, 1104-1110, DOI:10.1039/C5NJ02427G + cover picture - Slavik, P.; Kohout, M.; Böhm, S.; Eigner, V.; Lhotak, P.:
Synthesis of Inherently Chiral Calixarenes via Direct Mercuration of the Partial Cone Conformation
Chem. Commun. 2016, 52, 2366-2369 DOI:10.1039/C5CC09388K - Stejskal, F.; Curinova, P.; Lhotak, P.:
Unexpected Formation of Disulfide-based Biscalix[4]arenes
Tetrahedron 2016, 72, 760-766 DOI:10.1016/j.tet.2015.12.037
2015
- Kotek, V.; Dvořáková, H.; Tobrman, T.:
Modular and Highly Stereoselective Approach to All-Carbon Tetrasubstituted Alkenes
Org. Lett. 2015, 17, 608-611. DOI: 10.1021/ol503624v - Hartman, T.; Šturala, J.; Cibulka, R.:
Two-phase Oxidations with Aqueous Hydrogen Peroxide Catalysed by Amphiphilic Pyridinium and Diazinium Salts
Adv. Synth. Catal. 2015, 357, 3573-3586. DOI:10.1002/adsc.201500687 - Kolaříková, V.; Šimůnek, O.; Rybáčková, M.; Cvačka, J.; Březinová, A.; Kvíčala, J.:
Transition Metal Complexes Bearing NHC Ligands with Secondary Polyfluoroalkyl Groups
J. Chem. Soc., Dalton Trans. 2015, 44, 19663-19673. DOI:10.1039/C5DT02258D - Králík, A.; Linhart, I.; Váňa, L.; Moulisová, A.:
Identification of New DNA Adducts of Phenylnitrenium
Chem. Res. Toxicol. 2015, 28, 1317-1325. DOI:10.1021/acs.chemrestox.5b00120 - Kozmík, V.; Poznik, M.; Svoboda, J.; Frere, P.:
Dithieno[3,2-b:2 ',3 '-d]furan as a New Building Block for Fused Conjugated Systems
Tetrahedron Lett. 2015, 56, 6251-6253. DOI:10.1016/j.tetlet.2015.09.107 - Nunes, S.; Bürglová, K.; Hodačová, J.; Ferreira, R.; Carlos, L.; Almeida, P.; Cattoën, X.; Man, M.; Bermudez, V.:
Nanostructuring of Bridged Organosilane Precursors with Pendant Alkyl Chains
Eur. J. Org. Chem. 2015, 1218-1225. DOI:10.1002/ejic.201402673 - Zajicova, M.; Eigner, V.; Budka, J.; Lhotak, P.:
Intramolecular Bridging of Calix[4]arene Dialdoximes
Tetrahedron Lett. 2015, 56, 5529–5532 DOI:10.1016/j.tetlet.2015.08.032
- Vrzal, L.; Kratochvilova-Simanova, M.; Landovsky, T.; Polivkova, K.; Budka, J.; Dvorakova, H.; Lhotak, P.:
Application of RDC Enhanced NMR Spectroscopy in Structural Analysis of Thiacalix[4]arene Derivatives
Org. Biomol. Chem. 2015, 13, 9610-9618 DOI:10.1039/C5OB01424G - Stejskal, F.; Eigner, V.; Dvorakova, H.; Curinova, P.; Lhotak, P.:
Direct C-H Azidation of Calix[4]arene as a Novel Method to Access meta Substituted Derivatives
Tetrahedron Lett. 2015, 56, 5357-5361 DOI:10.1016/j.tetlet.2015.08.002 - Slavik, P.; Eigner, V.; Lhotak, P.:
Intramolecularly Bridged Calix[4]arenes with Pronounced Complexation Ability toward Neutral Compounds
Org. Lett. 2015, 17, 2788-2791 DOI:10.1021/acs.orglett.5b01200 - Liska, A.; Flidrova, K.; Lhotak, P.; Ludvik, J.:
Influence of Structure on Electrochemical Reduction of Isomeric Mono- and Di-, Nitro- or Nitrosocalix[4]arenes
Monatshefte fuer Chemie 2015, 146, 857-862 DOI:10.1007/s00706-015-1441-8 - Hucko, M.; Dvorakova, H.; Eigner, V.; Lhotak, P.:
2,14-Dithiacalix[4]arene and its Homooxa Analogues: Synthesis and Dynamic NMR Study of Conformational Behaviour
Chem. Comm. 2015, 51, 7051-7053 DOI:10.1039/C5CC00819K - Spendlikova, I.; John, J.; Cuba, V.; Jirasek, J.; Lhotak, P.:
Thiacalixarenes: Radiation Stability and Eu/Am Extraction in Synergistic Systems with COSANs
J. Radioanalyt. Nucl. Chem. 2015, 304, 257-262 DOI:10.1007/s10967-014-3694-9 - Mackova, M.; Miksatko, J.; Budka, J.; Eigner, V.; Curinova, P.; Lhotak, P.:
Chiral Anion Recognition by a Ureido-Thiacalix[4]arene Ligand Immobilized in the 1,3-Alternate Conformation
New J. Chem. 2015, 39, 1382-1389 DOI:10.1039/C4NJ01956C - Flidrova, K.; Liska, A.; Ludvik, J.; Eigner, V.; Lhotak, P.:
Fullerene Recognition by 5-Nitro-11,17,23,29-tetramethylcalix[5]arene
Tetrahedron Lett. 2015, 56, 1535-1538 DOI:10.1016/j.tetlet.2015.02.016 - Skacel, J.; Budka, J.; Eigner, V.; Lhotak, P.:
Regioselective Friedel-Crafts Acylation of Calix[4]arenes
Tetrahedron 2015, 71, 1959-1965 DOI:10.1016/j.tet.2015.02.021 - Trišović, N.; Antanasijević, J.; Tóth-Katona, T.; Kohout, M.; Salamonczyk, M.; Sprunt, S.; Jákli, A.; Fodor-Csorba, K.:
Azo-containing asymmetric bent-core liquid crystals with modulated smectic phase
RSC Adv. 2015, 5, 64886-64891. DOI: 10.1039/c5ra09764a
- Kohout, M.; Bielec, B.; Steindl, P.; Trettenhahn, G.; Lindner, W.:
Mechanistic aspects of the direct C-acylation of cyclic 1,3-diones with various unactivated carboxylic acids
Tetrahedron 2015, 71, 2698-2707. DOI: 10.1016/j.tet.2015.03.037
- von Koschitzky, I.; Gerhardt, H.; Lämmerhofer, M.; Kohout, M.; Gehringer, M.; Laufer, S.; Pink, M.; Schmitz-Spanke, S.; Strube, C.; Kaiser, A.:
New insights into novel inhibitors against deoxyhypusine hydrolase from plasmodium falciparum: compounds with an iron chelating potential
Amino Acids 2015, 47, 1155-1166. DOI: 10.1007/s00726-015-1943-z
- Kohout, M.; Kozmík, V.; Slabochová, M.; Tůma, J.; Svoboda, J.; Novotná, V.; Pociecha, D.:
Bent-shaped liquid crystals based on 4-substituted 3-hydroxybenzoic acid central core
Liq. Cryst. 2015, 42, 87-103. DOI: 10.1080/02678292.2014.965232
- Moulisová, A.; Linhart, I.:
Preparation of cysteine adducts by regioselective ring-opening reactions of phenyloxirane
Heterocyclic Commun. 2015, 21, 61-65 DOI:10.1515/hc-2015-0042 - Rybáčková, M.; Hošek, J.; Šimůnek, O.; Kolaříková, V.; Kvíčala, J.:
Computational study of productive and non-productive cycles in fluoroalkene metathesis.
Beilstein J. Org. Chem. 2015, 11, 2150–2157. DOI:10.3762/bjoc.11.232 - Hošek, J.; Rybáčková, M.; Čejka, J.; Cvačka, J.; Kvíčala, J.:
Synthesis of heavy Fluorous Ruthenium Metathesis catalysts using the stereoselective addition of polyfluoroalkyllithium to sterically hindered diimines.
Organometallics 2015, 34(13), 3327-3334. DOI:10.1021/acs.organomet.5b00325
- Křováček M., Dvořák D.:
Synthesis of potentially biologically active 6-(1,3-butadiynyl)purines.
J. Heterocyclic Chem. 2015, 52, 40-47 DOI:10.1002/jhet.1938 - Bousa, D.; Jankovsky, O.; Sedmidubsky, D.; Luxa, J.; Sturala, J.; Pumera, M.; Sofer, Z.:
Mesomeric Effects of Graphene Modified with Diazonium Salts: Substituent Type and Position Influence its Properties
Chem. Eur. J. 2015, 21, 17728-17738. DOI:10.1002/chem.201502127 - Tomanová P., Šturala J., Buděšínský M., Cibulka R.:
A click chemistry approach towards flavin-cyclodextrin conjugates – bioinspired sulfoxidation catalysts,
Molecules 2015, 20, 19837-19848. DOI:10.3390/molecules201119667 - Holakovský R., März M., Cibulka R.:
Urea derivatives based on a 1,1'-binaphthalene skeleton as chiral solvating agents for sulfoxides,
Tetrahedron: Asymmetry 2015, 26, 1328-1334. DOI:10.1016/j.tetasy.2015.10.011 - Mojr V., Svobodová E., Straková K., Neveselý T., Chudoba J., Dvořáková H., Cibulka R.:
Tailoring Flavins for Visible Light Photocatalysis: Organocatalytic [2+2] Cycloadditions Mediated by a Flavin Derivative and Visible Light,
Chem. Comm. 2015, 51, 12036 – 12039. DOI:10.1039/C5CC01344E - Sofer, Z.; Jankovský, O.; Šimek, P.; Sedmidubský, D.; Šturala, J.; Kosina, J.; Mikšová, R.; Macková, A.; Mikulics, M.; Pumera, M.:
Insight into the Mechanism of the Thermal Reduction of Graphite Oxide: Deuterium-Labeled Graphite Oxide Is the Key,
ACS Nano 2015, 9, 5478-5485. DOI:10.1021/acsnano.5b01463 - Šturala J., Boháčová S., Chudoba J., Metelková R., Cibulka R.:
Electron-deficient Heteroarenium salts – an Organocatalytic Tool for Activation of Hydrogen Peroxide in Oxidations,
J. Org. Chem. 2015, 80, 2676-2699. DOI:10.1021/jo502865f - Cibulka, R.:
Artificial flavin systems for chemoselective and stereoselective oxidations (Microreview),
Eur. J. Org. Chem. 2015, 915-932. DOI:10.1002/ejoc.201403275 + Cover picture
