Projects
- Synthesis of ligands for the lecitin receptor DC-SIGN
- Synthesis of muscarinic receptor agonists
- Synthesis of ligands for G-quadruplex visualization
Synthesis of ligands for the lectin receptor DC-SIGN
DC-SIGN is a C-type lectin receptor found on the surface of immune cells, particularly dendritic cells and macrophages. The natural ligands of the DC-SIGN receptor are oligo- and polysaccharides rich in mannose and fucose.
One of the main functions of the DC-SIGN receptor is the recognition of carbohydrates present on the surface of various pathogens: viruses (e.g., HIV, Ebola, SARS-CoV-2), bacteria (M. tuberculosis, S. pneumoniae), fungi (C. albicans), and parasites (Leishmania). Upon pathogen binding, the pathogen is internalized into the dendritic cell, triggering an immune response. However, some pathogens (e.g., HIV) exploit the interaction with the DC-SIGN receptor to facilitate their spread throughout the host organism.
In addition, DC-SIGN can transmit modulatory signals that either stimulate or suppress the immune response, depending on the type of bound ligand. It also plays a role in cell adhesion.
Ligands that bind selectively to the DC-SIGN receptor could have several practical medical applications:
1. Targeted drug delivery to dendritic cells: Ligands that selectively interact with the DC-SIGN receptor can be used for the targeted delivery of siRNA, antigens, or immunomodulators via liposomes, dendrimers, or nanoparticles.
2. Immunotherapy: Targeting dendritic cells can help enhance anti-tumour immunity. Nanoparticles modified with a specific antigen could be used to "train" dendritic cells to recognize that antigen.
3. Antimicrobial agents: Selective inhibition of the DC-SIGN receptor may allow modulation of the immune response at early stages of infection and open the door to a new class of drugs for treating these diseases.
The carbohydrate-binding site of the DC-SIGN receptor is rather shallow and hydrophilic, which makes it a less promising target in medicinal chemistry. However, recent studies have shown that in addition to its primary carbohydrate-binding site, DC-SIGN also possesses several secondary binding sites that are accessible to small organic molecules (Figure 1).
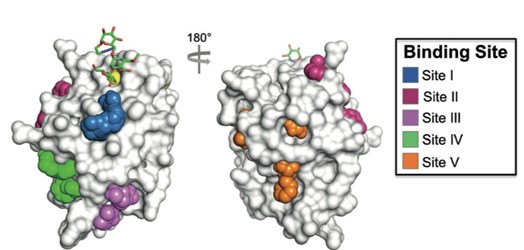
Figure 1. DC-SIGN receptor, primary carbohydrate binding site (yellow) and secondary binding sites I–V. Picture taken from Aretz, J. et al.: Angew. Chem. Int. Ed. 2017, 56, 7292–7296.
Our main goal is the synthesis of new DC-SIGN receptor ligands that exhibit high selectivity and favourable drug-like properties. We are preparing several types of ligands:
* Carbohydrate-based glycomimetics – compounds based on D-mannose or L-fucose, carrying substituents that interact either with the surroundings of the carbohydrate-binding site or with a secondary binding site (Figure 2a).
* Allosteric ligands – non-carbohydrate compounds that interact with secondary binding sites and, through their binding, modulate the interaction of carbohydrates with the primary carbohydrate-binding site (Figure 2b). The synthesis of these compounds follows a fragment-based approach.
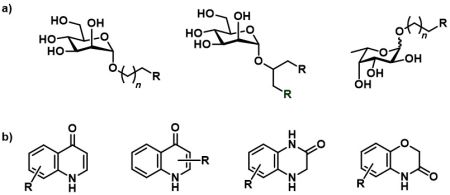
Figure 2. Overview of the synthesized DC-SIGN ligands. a) DC-SIGN inhibitors based on D-mannose and l-fucose; b) non-carbohydrate inhibitors binding to the secondary binding sites.
We collaborate on the project with the group of Prof. Christoph Rademacher at the University of Vienna. In his laboratory, compound affinity testing and nanoparticle formulation for targeted delivery are carried out.
Synthesis of muscarinic receptor agonists
Muscarinic acetylcholine receptors are G protein-coupled receptors that play a key role in many signaling pathways throughout the human body. The five known types of muscarinic receptors (M1–M5) are widely expressed in both the central and peripheral nervous systems. Each subtype can be involved in maintaining various physiological functions depending on the tissue. Achieving selectivity for a specific receptor subtype and signalling pathway is crucial in developing muscarinic receptor-targeted drugs in order to minimize potential side effects.
In 2020, a ligand based on a tetrahydropyridinium scaffold (Figure 3a) was discovered by Dr. Jakubík’s research group. This compound, which now serves as the lead structure for our project, showed selectivity for M2 muscarinic receptors and demonstrated so-called signalling bias toward the Gi/o pathway across all muscarinic receptor subtypes.
In this project, we focus on the synthesis of muscarinic receptor agonists based on the tetrahydropyridine scaffold, which hold potential for the treatment of neuropathic pain. The synthetic part of the project includes three main goals:
* Synthesis of analogues of our lead compound to study structure–activity relationships (SAR).
* Synthesis of bitopic ligands that incorporate both an orthosteric ligand (lead structure) and an allosteric ligand to enhance receptor affinity (Figure 3b).
* Synthesis of novel ligand types predicted using computer modelling techniques.
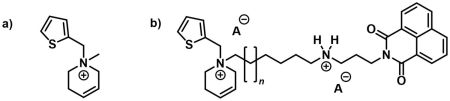
Figure 3. Examples of synthesized ligands for muscarinic receptors. a) Lead structure; b) example of a bitopic ligand.
We collaborate on this project with Dr. Jan Jakubík’s group at the Institute of Physiology of the Czech Academy of Sciences. In his laboratory, the affinity and selectivity of the compounds are tested.
Synthesis of ligands for G-quadruplex visualization
We focus on synthesizing ligands for the visualization of G-quadruplexes. Our goal is to develop twice-as-smart fluorescent probes that function both as smart G-quadruplex ligands (i.e., they are formed only in the presence of a natural G-quadruplex) and as smart fluorescent probes (i.e., they fluoresce only in the presence of their target, the G-quadruplex).
The first compound in this series (N-TASQ), synthesized by our collaborating group, was used for the very first visualization of G-quadruplexes in living human cells (Figure 4), and has since found
applications in oncology and neurology. Our aim is to prepare N-TASQ-related compounds and attempt to improve their properties.
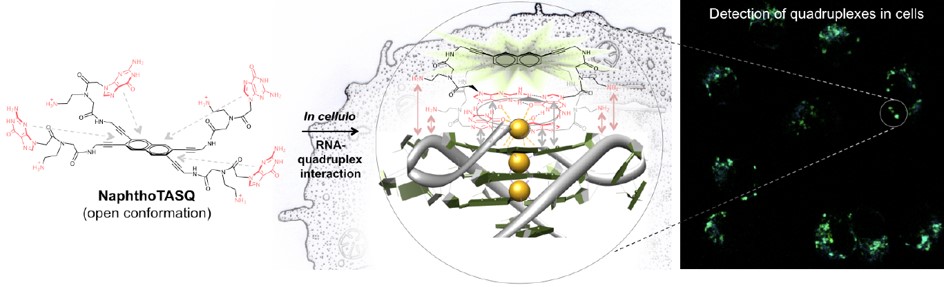
Figure 5. Twice-as-smart probes for the visualization of G-quadruplex. Picture taken from Laguerre, A. et al.: J. Am. Chem. Soc. 2015, 137, 8521–8525.
We collaborate on this project with Dr. David Monchaud’s group at ICMUB CNRS in Dijon, France. His laboratory conducts studies of the prepared compounds using biophysical methods.
